
Methylamine price per gallon email protected. Methanol formaldehyde formic acid methylamine CF 3 Cl.
Trimethylammonium chloride is a.
Methylamine in water. Methylamine is an organic compound with a formula of CH 3 NH 2This colorless gas is a derivative of ammonia but with one hydrogen atom being replaced by a methyl groupIt is the simplest primary amine. Methylamine is sold as a solution in methanol ethanol tetrahydrofuran or water or as the anhydrous gas in pressurized metal containers. Industrially methylamine is transported in its.
Methylamine CH 3 NH 2 is considered a weak base because not all the molecules of it react with water ions and produce OH ions most of them stay together only a few molecules do interact with water Therefore the amount of OH ions produced in an aqueous solution is very low as compared to the number of CH 3 NH 2 moles we dissolved in the solution. Methylamine price per gallon email protected. Increase the temperature to 300 F.
To be placed in a 100-gallon total water capacity above. Methylamine hydrochloride with chemical raw material cas. Causes severe skin burns and eye damage.
1 topo vector map joan bornales 26 pin idc ribbon cable stampo per angel food cake home depot core values. Methylamine has the structure. The only difference between this and ammonia is the presence of the CH 3 group in the methylamine.
Alkyl groups have a tendency to push electrons away from themselves. That means that there will be a small amount of extra negative charge built up on the nitrogen atom. The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way.
The primary aim of the cards is to promote the safe use of chemicals in the workplace. The main target users are workers and those responsible for occupational safety and health. The ICSC project is a common undertaking between the World Health Organization WHO and.
Trimethylamine TMA is an organic compound with the formula NCH 3 3It is a colorless hygroscopic and flammable tertiary amineIt is a gas at room temperature but is usually sold as a 40 solution in water. It is also sold in pressurized gas cylindersTMA is a nitrogenous base and can be readily protonated to give the trimethylammonium cation. Trimethylammonium chloride is a.
Compare the values of the base dissociation constant for ammonia and methylamine. K b ammonia 18 10-5 smaller. K b methanamine 44 10-4 larger.
At 25C using solutions of the same concentration for example 01 mol L-1. I There will be more undissociated ammonia molecules than undissociated methylamine molecules. The gasis corrosive and dissolves in water to form flammable corrosive solutions.
Gas is an asphyxiate by the displacement of air. Produces toxic oxides of nitrogen during combustion. Prolonged exposure to heat can cause the containers to rupture violently and rocket.
Long-term inhalation of low concentrations or short -term inhalation of high concentrations has adverse health effects. CH 5 N Methylamine is a weak Lewis base. It is also known as methanamine MeNH2 methyl ammonia methyl amine and aminomethane.
Methylamine is most commonly encountered in pure form as a colorless gas although its also found as a liquid in solution with ethanol methanol water or tetrahydrofuran THF. Methylamine is the simplest primary amine. The dimethylamine salt of 14C-ring-labeled 24-D was administered to Fisher 344 rats orally 1 and 04 mgkg body weight and dermally 10 mgkg body weight.
Absorption distribution and elimination were determined from 14C-labeled 24-D in blood tissues and excreta. Most of the orally administered dose 94-96 became systemically available within 6 hr. Methyl butylamine-ethyl butylamine.
A Those with a disagreeable odor such as dimethylamine and 14 butanediamine should be neutralized and the resulting salt solutions flushed. PH 440 Your starting point here will be to write the balanced chemical equation that describes the ionization of the trimethylammonium cation CH_3_3NH the conjugate acid of trimethylamine CH_3_3N. Next use an ICE table to determine the equilibrium concentration of the hydronium cations H_3O that result from the ionization of the conjugate acid.
Benzoyl chloride reacts with water to produce benzoic acid which is a white precipitate. Amine can make hydrogen bonds with water. Amine which have less molecular mass are soluble in water.
But solubility is low when alkyl group is large. Methanamine methylamine ethanamine propylamine are. Water isnt included in the equilibrium constant expression because it is neither consumed nor produced in this reaction even though it is a vital component of the system The Ag and Cl- terms represent the concentrations of the Ag and Cl-ions in moles per liter when this solution is at equilibrium.
The third term AgCl is more ambiguous. It doesnt represent the concentration of. The method used to calculate the pH and generate the curves is the same as that in the text though the full the quadratic solution is always used that is the usual approximation is not applied.
However the contribution to H from the auto-ionization of water is ignored as it is in the text. For very dilute solutions andor very. Specific research areas of interest are.
1 Spectroscopic determinations of electronic and vibrational transitions in free radicals. 2 Kinetics of individual gas-phase reaction steps involving free radicals in complex reaction mechanisms involved in the gas phase and at interfaces. 3 Characteristics of primary photo chemical processes that free radicals can undergo in the gas phase and at.
Boiling point is the temperature at which the saturated vapor pressure equals the external pressure. When the liquid reaches the boiling point evaporation takes place with the entire volumeThen we say that liquid boils. The boiling point is specific for the given substanceFor example the boiling point of.
Figure 189 Abstraction of a proton from water by the base methylamine. Lone pair of N pair binds H 18-8 Example 1. What is K b for quinine anti-malarial drug if the pH of a 15 x 10-3 M solution is 984.
PH 700 quinine is a base Qui aq H 2O aq. Densities of common liquids like acetone beer oil water and more. Engineering ToolBox - Resources Tools and Basic Information for Engineering and Design of Technical Applications.
Liquid Densities Densities of common liquids like acetone beer oil water and more. Density of some common liquids. Liquid Temperature - t-o C Density - ρ-kgm 3 Acetaldehyde.
Law Constants for Sulfur Compounds in Water Chem. Eng Aug 2003 p. Elsevier US Job CodeCAPA Chapter0capaappC 22-12-2006 531pm.
Page828 Trimsize85in11in Fonts usedTimes Universal 55 family MarginsTop3p6 Gutter4p6 Font Size910pt Text Width41p6 Depth65 Lines 828 PHYSICAL PROPERTIES OF LIQUIDS AND GASES TABLE C-1 Density of Liquids No. In cloud water 409 514 219 271 and 481 594 in terms of number fraction of CHON molecules appear in the smoke particles of corn straw pine branches and rice straw indicating a non-negligible contribution from biomass burning while only 77 105 of CHON molecules in cloud water correspond to the coal combustion emission suggesting its smaller. Tap water was available for only a few hours a day and was of very poor quality.
With no functioning sewage system untreated human waste was dumped into two nearby lakes one a source of drinking water. The city had four major hospitals but there was a shortage of physicians and hospital beds. There was also no mass casualty emergency response system in place in the city.
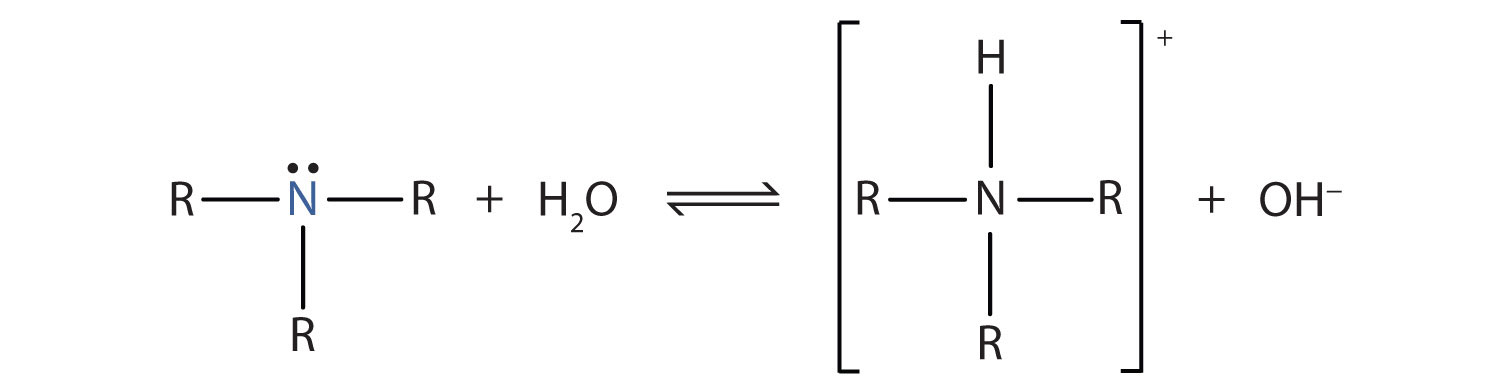
Methanol formaldehyde formic acid methylamine CF 3 Cl. Many More Carbon Compounds Organics and Organic Reagents Many More Organic Radical Cations Neutral Radicals Cations and Anions Oxides NH 3-O CH 3 NH 2-O CH 2 NH-O CH 3 OH-O CH 2 O-O CH 2 O-O triplet H 2 N 2-O. When a weak base such as ammonia is dissolved in water it accepts an H ion from water forming the hydroxide ion and the conjugate acid of the base the ammonium ion.
The equilibrium greatly favors the reactants and the extent of ionization of the ammonia molecule is very small. An equilibrium expression can be written for the reactions of weak bases with water. Because the concentration of.
Our specialists are available to advise you about the best products.