The mercury concentration was Hamilton syringe 31 dissolved in water three-time a week. It should be kept under kerosene to prevent oxidation.
The finely divided metal ignites spontaneously in air.
Mercuric oxidation in water. MercuryII oxide also called mercuric oxide or simply mercury oxide has a formula of Hg O. It has a red or orange color. MercuryII oxide is a solid at room temperature and pressure.
The mineral form montroydite is very rarely found. Our product line consists of chemical solutions prepared to exact quality standards and certified for use in laboratories and production processes. We regularly produce chemical solutions to specifications designed by government and regulatory bodies commercial and trade associations and the specific needs of individual users and businesses.
It is a fast applicable parameter for industrial wastewater water controlling plant sewage rivers lakes or aquifers but not applicable for drinking water as the lower content of oxidizable organic matter. Frequently a silver compound like Silver sulfate is used as a catalyst to promote oxidation of certain organic compounds such as linear aliphatic compounds aromatic compounds and. A typical reagent for this process is mercuric oxide.
RHN 2 CS. EDC is a water-soluble carbodiimide reagent used for a wide range of purposes. Apart from uses similar to those of DCC and DIC it is also used for various biochemical purposes as a crosslinker or chemical probe.
1-cyclohexyl-2-morpholinoethylcarbodiimide metho-p-toluene sulfonate is a. Where there is oxidation there is always reduction Chemistry is essentially a study of redox systems. 264 CHEMISTRY In reactions 81 and 82 the elements magnesium and sulphur are oxidised on account of addition of oxygen to them.
Similarly methane is oxidised owing to the addition of oxygen to it. CH4 g 2O2 g CO2 g 2H2O l 83 A careful examination of. Animals in group III perchloric acid 2v1v in boiling water bath until evaporation.
Were administered a single dose 5 mgkg bw of mercury II After digestion the samples were diluted by definite volume of chloride HgCl2 by subcutaneous sc route with the help of distilled water and filtered. The mercury concentration was Hamilton syringe 31 dissolved in water three-time a week. The general base B used in these mechanisms may be anything from water to pyridine depending on the specific reaction.
Two structural requirements for the oxidation to carbonyl products should now be obvious. The carbon atom bonded to oxygen must also bear a hydrogen atom. Tertiary alcohols R 3 COH cannot be oxidized in this fashion.
The oxygen atom must be bonded to a hydrogen. For instance the oxidation state of carbon atoms in the wood increases during the combustion of wood with molecular oxygen and that of oxygen atoms decreases as carbon dioxide and water are produced. The oxygen atoms are reduced formally receiving electrons while the carbon atoms are oxidised losing electrons.
Therefore oxygen is the oxidising agent and the reducing agent in this. The Water Analysis Handbook WAH is the result of more than 85 years of research and method development. With over 300 illustrated step-by-step instructions this is your comprehensive source for water analysis procedures.
From instruments to reagents meters to probes media to general lab supply this handbook outlines everything you need to perform each procedure simplifying the water. 1 2 2 in compounds with fluorine electron config. 1s 2 2s 2 2p 4.
Oxygen was discovered about 1772 by a Swedish chemist Carl Wilhelm Scheele who obtained it by heating potassium nitrate mercuric oxide and many other substances. An English chemist Joseph Priestley independently discovered oxygen in 1774 by the thermal decomposition of mercuric oxide. OxidationReduction Potential Measurement in Clean Water 2710 TESTS ON SLUDGES A.
Settled Sludge Volume D. Sludge Volume Index E. Zone Settling Rate F.
Capillary Suction Time H. Modified Settled Sludge Volume 2720 ANAEROBIC SLUDGE DIGESTER GAS ANALYSIS. Alkenes can be converted to alcohols by the net addition of water across the double bond.
There are multiple ways that are commonly used to do this transformation. The net addition of water to alkenes is known as hydration. The result involves breaking the pi bond in the alkene and an OH bond in water and the formation of.
Ferrous 110-phenanthroline an oxidation-reduction indicator changes from red to pale blue when the oxidation potential of the solution is increased from 104 to 108 volts. And diphenylcarbazone an indicator for mercuric ion changes from yellow to violet when the mercuric ion concentration is increased from 0000001 to 000001 mole per litre. Each of these indicators thus has a relatively.
Barium chloride forms a crystalline hydrate BaCl 2 xH 2 O in which x molecules of water are incorporated into the crystalline solid for every unit of BaCl 2. This water can be driven off by heat. If 110 g of the hydrated salt is heated and reweighed several times until no further loss of weight ie loss of water occurs the final weight of the sample is 0937 g.
What is the value of. Introduction Chemical oxygen demand COD is defined as the amount of a specified oxidant that reacts with the sample under controlled conditions. The quantity of oxidant consumed is expressed in terms of its oxygen equivalence.
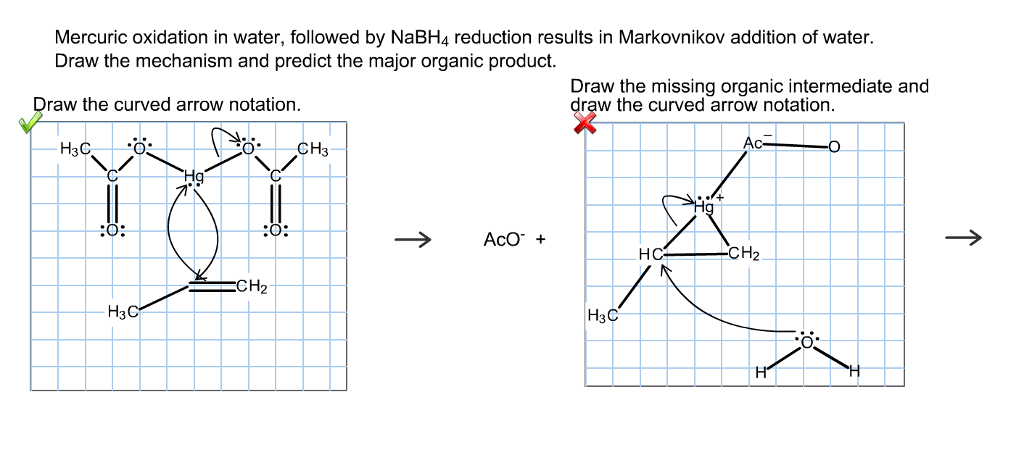
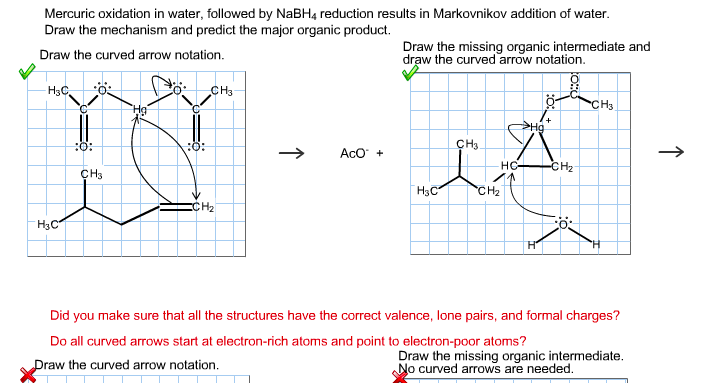
Because of its unique chemical properties the dichromate ion Cr2O72 is the specified oxidant in Methods 5220B C and D. It is reduced to the chromic ion. Alkenes can be converted to alcohols by reaction with mercuric acetate to form a β-hydroxyalkylmercuryII acetate compound a reaction called oxymercuration.
Subsequent reduction with NaBH4 reduces the C-Hg bond to a C-H bond forming the alkyl alcohol a reaction called demercuration. Draw the structure of the Hg-containing compound and the final alcohol product formed in the. DR3900 Laboratory Spectrophotometer for water analysis Order Status Contact Us Login.
Cart 0 Quote. Mercuric Thiocynanate Method 8113 DOC3165301017. Chlorine Dioxide Direct Reading Method 8138 DOC3165301019.
Chlorine Dioxide DPD Method 10126 Powder Pillows or AccuVac Ampuls DOC3165301021. We would like to show you a description here but the site wont allow us. Hydrargyrum comes from Greek words for water-silver hydr-means water argyros means silver.
Mercury is a very rare element in the Earths crust. It accounts for only about 008 parts per million ppm and is mainly found in the mineral cinnabar which is mercuric sulfide. Mercuric sulfide is the source of the red pigment called vermilion.
Water the sample cannot be preser ved and It Is necessary to carry out analysl8 on sit. 24 h 24 h Analyse soon as poalble 24 h Analyses soon as posaible e. 7 days Analya aoon a poaslble On alte 7 days e h 100 500 500 5 500 500 100 1100 300 000 4 Add mercuric chloride 40.
The addition of a small amount of mercuric chloride will amalgamate the surface of the metal allowing it to react. Industrial production Grignard reagents are produced in industry for use in place or for sale. As with at bench-scale the main problem is that of initiation.
A portion of a previous batch of Grignard reagent is often used as the initiator. Grignard reactions are exothermic. Mercury also exists as a cation with oxidation states of 1 mercurous or 2 mercuric.
Methylmercury is the most frequently encountered compound of the organic form found in the environment and is formed as a result of the methylation of inorganic mercuric forms of mercury by microorganisms found in soil and water 209. Since the implementation of the Clean Water Act and subsequent creation of the United States. Pre-prepared vials that change color from orange to green based on the amount of oxidation and that are read using a laboratory colorimeter.
Prior to completing the COD test a series of known standards are prepared using KHP potassium hydrogen phthalate. Standard Methods For the Examination of Water and Wastewater 23nd edition. Standard Methods For the Examination of Water and Wastewater 23nd edition 2017.
Full PDF Package Download Full PDF Package. A short summary of this paper. 32 Full PDFs related to this paper.
Standard Methods For the Examination of Water and Wastewater. Strontium is softer than calcium and decomposes in water more vigorously. It does not absorb nitrogen below 380oC.
It should be kept under kerosene to prevent oxidation. Freshly cut strontium has a silvery appearance but rapidly turns a yellowish color with the formation of the oxide. The finely divided metal ignites spontaneously in air.