It also depletes the antioxidant glutathione according to rat and monkey studies 20 23 4. ManganeseII can form the MnIIHDFOB complex this undergoes oxic oxidation to MnIIIDFOB.

American Manganese Successfully Completes All Identified Milestones in the DLA Project Scope of Work Within the Project TimelineAmerican Manganese Inc.
Manganese and oxygen form. All natural manganese is the stable isotope manganese-55. It exists in four allotropic modifications. The complex cubic structure of the so-called alpha phase is the form stable at ordinary temperatures.
Manganese somewhat resembles iron in general chemical activity. The metal oxidizes superficially in air and rusts in moist air. It burns in air or oxygen at elevated temperatures as does iron.
Manganese is essential to iron and steel production by virtue of its sulfur-fixing deoxidizing and alloying properties as first recognized by the British metallurgist Robert Forester Mushet 18111891 who in 1856 introduced the element in the form of Spiegeleisen into steel for the specific purpose of removing excess dissolved oxygen sulfur and phosphorus in order to improve its. Typically a controlled amount of zincii nitrate hexahydrate and manganeseiii acetate dihydrate were dissolved into DMF to form a uniform solution in a round-bottom flask A. You will find the reactions between hexaaqua ions and hydroxide ions discussed in detail if you follow this link.
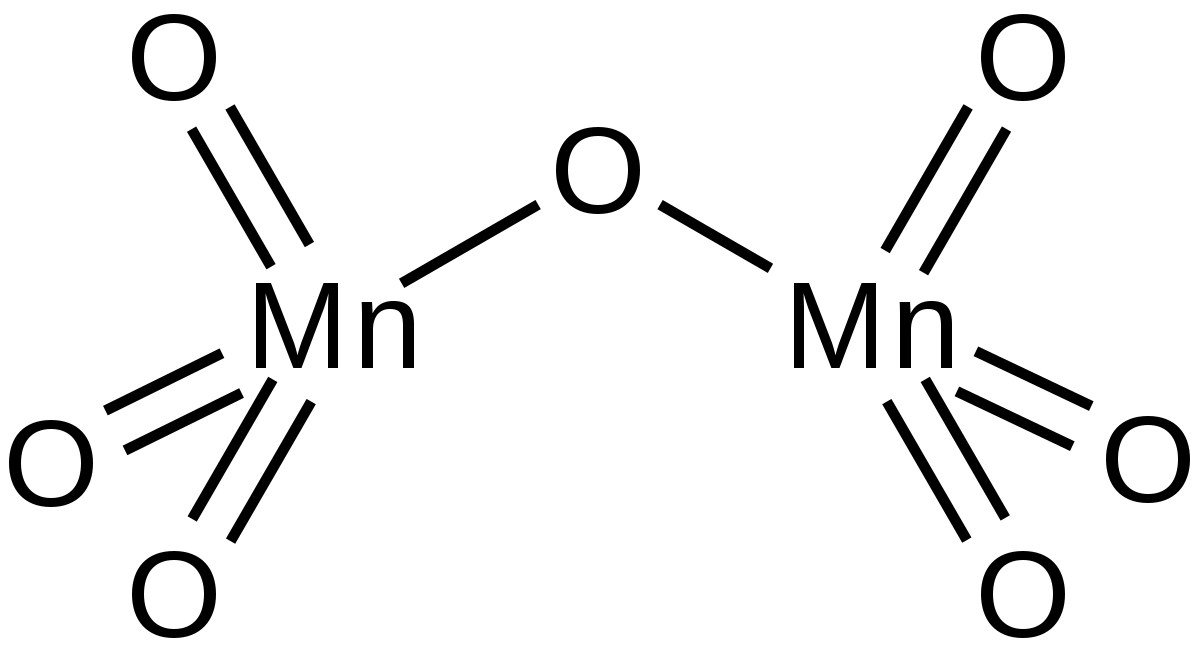
There is a lot of disagreement in the literature about what the darker brown compound is. It is most commonly quoted including in a couple of research papers as MnOOH or sometimes as Mn 2 O 3xH 2 O. However some sources quote it as a hydrated manganeseIV oxide MnO 2.
2 catalyses several reactions that form O 2. In a classical laboratory demonstration heating a mixture of potassium chlorate and manganese dioxide produces oxygen gas. Manganese dioxide also catalyses the decomposition of hydrogen peroxide to oxygen and water.
2 H 2 O 2 2 H 2 O O 2. Manganese dioxide decomposes above about 530 C to manganeseIII oxide and oxygen. Elemental manganese readily combines with oxygen carbon and silicon to form a long list of manganese minerals.
Manganese ores generally contain 25 to 45 percent manganese mostly in oxide or hydroxide and carbonate minerals. Manganese ores are widespread but most of the worlds supply is from a small number of manganese mining districts. Most manganese ores are from extensive layers.
Manganese in the form of the black ore pyrolucite manganese dioxide. It is the star player in the reaction centre of photosystem II where water molecules are converted to oxygen. Without manganese there would be no photosynthesis as we know it and there would be no oxygen in the atmosphere.
While biology discovered manganese early on it took humankind a bit longer. Already in ancient. Manganese is a grey-white hard and very brittle metal.
It tarnished on exposure to air and oxidized to manganese when heated. Certain Facts About Manganese. Manganese is more reactive when available in pure form and as a powder it will burn in oxygen to react with water and dissolves in dilute acids.
Manganese mix with dissolved oxygen. These bacteria form thick slime growths on the walls of the piping system and on well screens. These shines tend to be are rust-colored from iron and black-colored from manganese.
Variations in flow can cause these slime growths to separate from pipe walls resulting in dirty water in the system. The growth of iron bacteria can be controlled by chlorination. MANGANESE APPLICATIONSVacuum induction melted alloys.
Various ferrous and non-ferrous speciality alloys with stringent purity controls are produced with MMCs electrolytic Mn due to the low levels of hydrogen lead bismuth and the absence of selenium. Contamination of these high-value alloys is extremely costly but MMCs quality controls and full product traceability provides peace of. In addition to the concentration it is also important to determine the form of the iron and manganese.
If water collected from the well or spring is initially clear but then forms orange-brown or black solid particles over time the iron and manganese are dissolved in the water. This is known as the reduced form of these metals. Dissolved or reduced iron and manganese are most common in.
Oxygen vacancy on the heterogeneous catalyst is of great importance to the catalysis of volatile organic compound VOC oxidation. Herein microwave radiation with special energy-excitation is successfully utilized for the post-processing of a series of manganese oxides MnO x to generate oxygen vacanciesIt is found that the MnO x catalyst with 60 min of microwave radiation demonstrates. The Antarctic bottom water for example transports oxygen-rich water from the sea surface to greater depths.
Without this the manganese oxide compounds could not form. The sediment has to be capable of holding large amounts of pore water. Diagenetic nodule growth can only take place in very aqueous sediments.
213 Manganese nodules are present in. Manganese is a chemical element with atomic number. An atom of Oxygen in the gas phase for example gives off energy when it gains an electron to form an ion of Oxygen.
O e O H Affinity 141 kJmol. To use electron affinities properly it is essential to keep track of sign. When an electron is added to a neutral atom energy is released.
This affinity is known as. ManganeseII can form the MnIIHDFOB complex this undergoes oxic oxidation to MnIIIDFOB. The formation of MnII.
Rapid oxygen-dependent microbial MnII oxidation kinetics at sub-micromolar oxygen concentrations in the Black Sea suboxic zone. Acta 73 2009 pp. Article Download PDF View Record in Scopus Google Scholar.
ManganeseII sulfate is a metal sulfate in which the metal component is manganese in the 2 oxidation state. It has a role as a nutraceutical. It is a metal sulfate and a.
In excess manganese builds up in mitochondria and increases the production of reactive oxygen species. It also depletes the antioxidant glutathione according to rat and monkey studies 20 23 4. Oxygen is a chemical elementIt has the symbol O and atomic number 8.
It is the third most common element in the universe after hydrogen and heliumOxygen is more than a fifth of the Earths atmosphere by volume. In the air two oxygen atoms usually join to make dioxygen O 2 a colourless gasThis gas is often just called oxygen. It has no taste or smellIt is pale blue when it is liquid or.
Oxygen 995 pure is blown into the BOF at supersonic velocities. It oxidizes the carbon and silicon contained in the hot metal liberating great quantities of heat which melts the scrap. There are lesser energy contributions from the oxidation of iron manganese and phosphorus.
A big difference between the two steelmaking processes however is in the capital investment costs involved. Oxygen accounts for about 23 of the atmospheres mass with pairs of oxygen atoms stuck together to make dioxygen molecules but its not just in the air we breathe. Overall its the most abundant element on the earths surface and the third most abundant in the universe after hydrogen and helium.
Our planets rocks are about 46 oxygen by weight much of it in the form of silicon dioxide. Use an oxidizing iron filter such as Pro-OX or other manganese dioxide iron filters to turn the dissolved iron to rust form where it is then filtered out by the iron filter. Periodic backwash keeps the Pro-OX filter media clean.
Often these iron filters use aeration to enhance the oxidizing ability of the filter media. Also known as ferric iron rust this is iron that has. This is an excited form of oxygen in which one of the electrons jumps to a superior orbital following absorption of energy.
Formation of Reactive Oxygen Species. Oxygen-derived radicals are generated constantly as part of normal aerobic life. They are formed in mitochondria as oxygen is reduced along the electron transport chain.
Reactive oxygen species are also formed as necessary. Potassium in cantaloupe promotes the supply of oxygen to the brain making one feel relaxed and focused. It contains superoxide dismutase a compound which combats stress and calms anxieties.
It soothes the nervous system preventing sleeping disorders. It also prevents cell death caused by oxidative stress. Cantaloupe juice is very effective in regulating menstrual flow and.
The dissolved oxygen in the sample is then fixed by adding a series of reagents that form an acid compound that is then titrated with a neutralizing compound that results in a color change. The point of color change is called the endpoint which coincides with the dissolved oxygen concentration in the sample. Dissolved oxygen analysis is best done in the field as the sample will be less.
American Manganese Successfully Completes All Identified Milestones in the DLA Project Scope of Work Within the Project TimelineAmerican Manganese Inc.