As KOH dissociates into K and OH this OH ion accepts the proton H to become water. The resulting solution is basic because of the dissolved hydroxide.
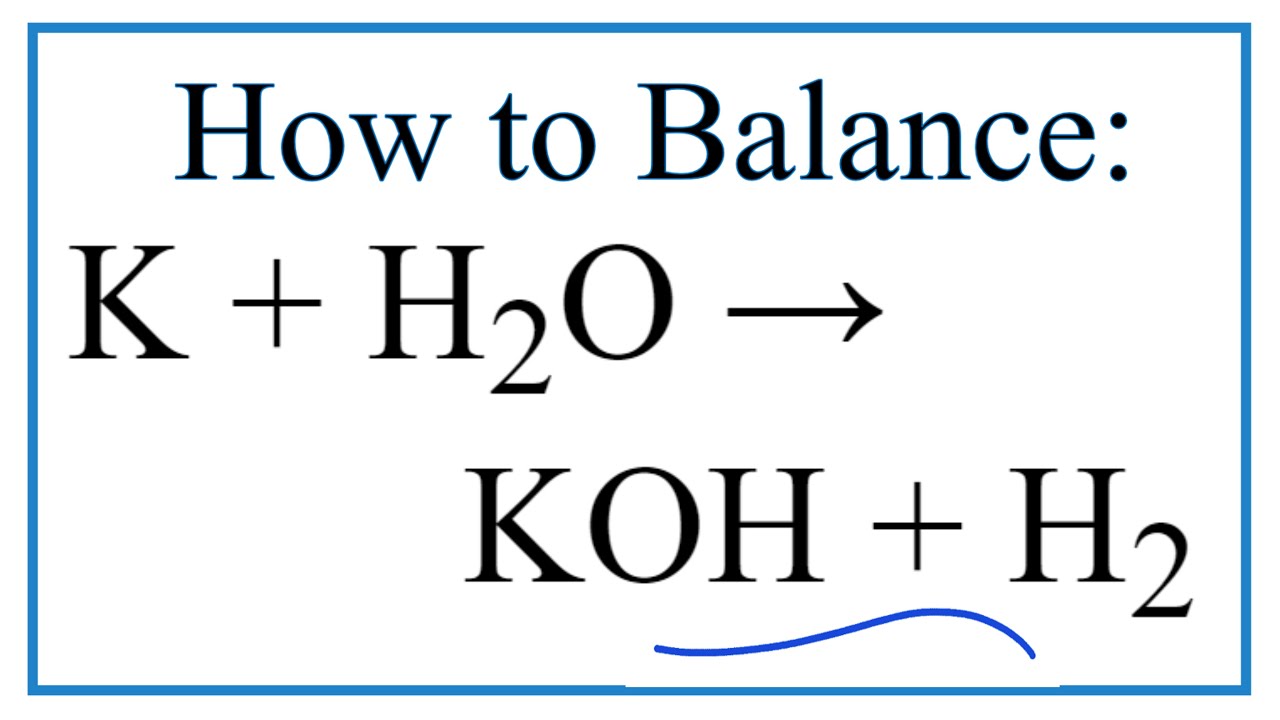
Potassium metal reacts very rapidly with water to form a colourless solution of potassium hydroxide KOH and hydrogen gas H 2.
Koh dissolves in water. A base is defined as a proton acceptor or lone pair donor. When KOH dissolves in water is split into two ions K and OH. As KOH dissociates into K and OH this OH ion accepts the proton H to become water.
KOH aq K aq OH aq Theoretically we have three concepts to check whether KOH is acid or base. Bronsted-Lowry concept c. Pour the KOH into the water and glycerine mixture.
Not the other way around Mix well until the KOH fully dissolves into the waterglycerine. It will heat up and be cloudy at first but will clear up. Carefully pour the KOH mixture into the warm coconut oil and slowly mix them together.
This can be done in the slow cooker or in a large bowl if you dont have a slow cooker. Using a hand. Water and alcohol are removed to produce 80-88 pure glycerin that is ready to be sold as crude glycerin.
In more sophisticated operations the glycerin is distilled to 99 or higher purity and sold into the cosmetic and pharmaceutical markets. Methyl Ester Wash Once separated from the glycerin the biodiesel is sometimes purified by washing gently with warm water to remove residual catalyst. 1 g Potassium hydroxide KOH in 100 ml water at room temperature.
Antimony Etchant Etch off of silicon. 3 90110 Aqua Regia HCl. 3 31 Evaporation - removal.
50 DI water. Never store in a tightly sealed container. Bismuth Etchant 5 ml Sulfuric acid.
5 ml Hydrogen peroxide. Sugar dissolves in water because energy is given off when the slightly polar sucrose molecules form intermolecular bonds with the polar water molecules. The weak bonds that form between the solute and the solvent compensate for the energy needed to disrupt the structure of both the pure solute and the solvent.
In the case of sugar and water this process works so well that up to 1800 grams of. A weak acid only partially dissociates in water to give H and the anion. Examples of weak acids include hydrofluoric acid HF and acetic acid CH 3 COOH.
Molecules that contain an ionizable proton. A molecule with a formula starting with H usually is an acid. Organic acids containing one or more carboxyl group -COOH.
The H is ionizable. Anions with an ionizable proton. In the presence of water or simply ambient moisture the strong bases NaOH or KOH readily dissolve in their hydration water hygroscopic substances deliquescence phenomenon and this greatly facilitates the catalysis process because the reaction in aqueous solution occurs much faster than in the dry solid phase.
The moist NaOH impregnates the surface and the porosity of calcium hydroxide. So based on arrhenius theory acid is the substance which ionizes when it dissolves in the water solution. It will produce the hydrogen ion H.
While the base is the substance which produces hydroxide ion OH- when it dissolves in the water solution. The example of acids are HCl HCN H2SO4. The reaction of acid for example HCl solution is explained as follow.
HClg H aq Cl. A solute dissolves in excess solvent to form a solution. Solute solvent solution.
Heat of solution or. NH 4 C 2 H 3 O 2 s-238. NaC 2 H 3 O 2 s-1732.
KC 2 H 3 O 2 s-1533. Do you know this. Play the game now.
Experiment to Determine the Molar Heat of Solution Molar Enthalpy of Solution of a Solute. The following describes the use of. Reaction of potassium with water.
Potassium metal reacts very rapidly with water to form a colourless solution of potassium hydroxide KOH and hydrogen gas H 2. The resulting solution is basic because of the dissolved hydroxide. The reaction is exothermic.
Early in the reaction the potassium metal becomes so hot that it catches fire and burns with a characteristic pale lilac colour. Potassium hydroxide also known as lye is an inorganic compound with the chemical formula KOHAlso commonly referred to as caustic potash it is a potent base that is marketed in several forms including pellets flakes and powdersIt is used in various chemical industrial and manufacturing applications. Potassium hydroxide is also a precursor to other potassium compounds.
POTASSIUM HYDROXIDE SOLUTION is a strong base dissolved in water. Reacts exothermically with all acids. Attacks aluminum and zinc to generate flammable hydrogen gas.
Ignited a polyethylene container liner when mixed with potassium persulfate by release of heat and oxygen MCA Case History 1155. The substance in the smallest amount and the one that dissolves or disperses is called the SOLUTE. The substance in the larger amount is called the SOLVENT.
In most common instances water is the solvent. The gases liquids or solids dissolved in water are the solutes. In the graphic the blue bottle is a homogeneous solution mixture of water KOH glucose oxygen gas dissolved and methylene.
The wrappers are actually made of edible rice cellophane that dissolves in your mouth. Why bother unwrapping when we can eat them altogether. The fancy shiny foils that were wrapped around the chocolate fingers never failed to attract the kids attention.
One of the simple pleasures Malaysians had. Popo Fish Muruku. A pioneer among snacks Popo.
Its chemical formula is KOH. It contains potassium and hydroxide ions. It is a white powder.
It dissolves easily in water to make a basic solution. It is corrosive to skin. It reacts with acids to give potassium salts.
It is less common than sodium hydroxide. About 700000 tons are made each year. It is hygroscopic meaning that it absorbs water from the air and forms a solution.
A wide range of chemicals have been tested as deproteinization reagents including NaOH Na 2 CO 3 NaHCO 3 KOH K 2 CO 3 CaOH 2 Na 2 SO 3 NaHSO 3 CaHSO 3 Na 3 PO 4 and Na 2 S. Reactions conditions vary considerably in each study. NaOH is the preferential reagent and it is applied at concentration ranging from 0125 to 50 M at varying temperature up to 160 C and treatment.
But oxygen dissolves in water easily than Nitrogen hence water contains a higher portion of oxygen. F If distilled water is kept in a sealed bottle for a long time it leaves etchings on the surface of the glass because substances that are insoluble in water dissolve in trace amounts even small amounts of glass dissolves in water which will make etching on the glass surface. G Rainwater does.
Lithium gradually reacts with water and disappears forming a colorless solution of lithium hydroxide LiOH. The reaction generates heat too slowly. 2Li 2H 2 O 2LiOH H 2 Sodium and water reaction.
As lithium sodium also floats on the surface but enough heat is generated to melt the sodium metal melting point of sodium is lower than lithium and the reaction produces heat faster and. For example ceKOH dissolves in water in the reaction ceKOH rightarrow K OH- nonumber Relative to the number of strong acids there are fewer number of strong bases and most are alkali hydroxides. Calcium hydroxide is considered a strong base because it is completely almost completely ionized.
However the solubility of calcium hydroxide is very low. The 18-piece set includes 12 Goldfaber Aqua Pencils that offer excellent lightfastness and smooth color laydown that dissolves in with water to reveal vibrant colors. Faber-Castell Goldfaber Aqua Gift Set.
2 out of 5 stars 1 Reviews Item. The Faber-Castell Goldfaber Aqua Gift Set is the perfect gift for any professional watercolorist or artist looking to begin. KOH AgBr CaCl 2 PbNO 32 PbSO 4 KOH is soluble because it contains K AgBr is insoluble.
Most bromides are soluble but Ag Br is an exception CaCl 2 is soluble. Most chlorides are soluble and Ca Cl 2 is not an exception PbNO 32 is soluble because it contains NO 3 PbSO 4 is insoluble. Most sulfates are soluble but Pb SO 4 is.
KOH with K and OH-ions. NH 4 2 CO 3 with NH 4 and CO 3 2-ions. Although they consist of positively and negatively charged ions ionic compounds are electrically neutral because the charges are always equal and opposite.
The other major group of compounds is molecular compounds. These consist of molecules rather than ions Bonding in Ionic. Note that while calcium hydroxide barium hydroxide and strontium hydroxide are strong bases they are not very soluble in water.
The small amount of compound that dissolves dissociates into ions but most of the compound remains a solid. KOHs K aqOH-aq 56 kJmol LiBrs Li aqBr. When HClaq dissolves in NaOHaq forming Na aqCl-aq 57 kJmol are.
Released 57 kJmol of heat of solution. Many other properties can be found in. For some special solutions.
Salt-water sugar-water alcohol-water hydrogen peroxide-water ammonia-water and carbon dioxide-water. Unlike the hydroxides of aluminum and lead zinc hydroxide also dissolves in excess aqueous ammonia to form a colorless water-soluble ammine complex. Zinc hydroxide will dissolve because the ion is normally surrounded by water ligands.
When excess sodium hydroxide is added to the solution the hydroxide ions will reduce the complex to a 2 charge and make it soluble. When excess ammonia is.