Potassium permanganate occurs in the form of monoclinic prisms almost opaque with a blue metallic lustre. Concepts covered in Elements of Groups 16 17 and 18 are Anomalous Behaviour of Fluorine Anomalous Behaviour of Oxygen Atomic and Physical Properties of Elements of Group 16 17 and 18 Chemical Properties of Elements of Groups 16 17 and 18 Chlorine Concept of Group 18 Elements Electronic Configuration of Elements of Group 16 17 and 18 Occurrence of Elements of Groups 16 17 and 18.
Oxoacids of Sulphur Structures only.
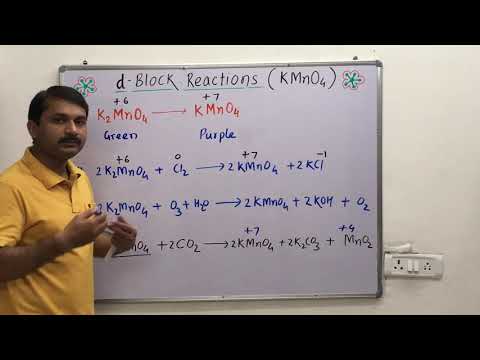
Kmno4 physical properties. Physical Properties Of Potassium Permanganate KMnO4. It is an odourless purple to magenta crystalline solid. It is soluble in water acetone acetic acid methanol and pyridine.
It gets dissolved in ethanol and organic solvents. Potassium permanganate occurs in the form of monoclinic prisms almost opaque with a blue metallic lustre. Physical Properties of Potassium Permanganate KMnO 4.
It is a bright purple or bronze coloured chemical compound. It has a density of 27gml and its molar mass is 158034gmol. The compound is odourless ie.
It has no smell but has a sweet taste. It has a high melting point of 240 0 C. It is mostly found in powder crystal or in tablet form.
Indicators are substances that change in physical appearance eg color at or approaching the endpoint of a chemical titration eg on the passage between acidity and alkalinity. Reagents are substances used for the detection or determination of another substance by chemical or microscopical means especially analysis. Types of reagents are precipitants solvents oxidizers reducers.
Alkenes contain a carbon-carbon double bond which changes the physical properties of alkenes. Alkenes are unsaturated carbon compounds which have a general formula of C_nH_2n. These compounds are also known as olefins.
Alkenes are a family of compounds containing hydrogen and carbon only hydrocarbons with a carbon-carbon double bond. Potassium permanganate is an inorganic compound with the chemical formula KMnO 4 and composed of K and MnO 4It is a purplish-black crystalline salt that dissolves in water to give intensely pink or purple solutions. Potassium permanganate is widely used in chemical industry and laboratories as a strong oxidizing agent and also as a medication for dermatitis for cleaning wounds and.
Houston Texas 77396 US Sales. 1-800-424-9300 International CHEMTREC call. 1-703-527-3887 For non-emergency assistance call.
These properties are due to metallic bonding by delocalized d electrons leading to cohesion which increases with the number of shared electrons. However the Group 12 metals have much lower melting and boiling points since their full d subshells prevent dd bonding. In fact mercury has a melting point of 3883 C 3789 F and is a liquid at room temperature.
General introduction electronic configuration oxidation states occurrence trends in physical and chemical properties dioxygen. Preparation properties and uses classification of Oxides Ozone Sulphur -allotropic forms. Preparation properties and uses of Sulphur-dioxide Sulphuric Acid.
Oxoacids of Sulphur Structures only. To investigate the physical properties solubility and density of some hydrocarbon. To compare the chemical reactivity of an alkane an alkene and an aromatic compound.
To use physical and chemical properties to identify an unknown. Introduction - Hydrocarbons are the compounds containing only the hydrogen and the carbon elements. The vast number of hydrocarbons is indicative of the.
Amphotericin B is a polyene antifungal antibiotic produced by Streptomyces nodosus with antifungal activity. Amphotericin B binds to ergosterol an essential component of the fungal cell membrane thereby causing depolarization of the membrane and altering cell membrane permeabilityThis leads to leakage of important intracellular components cell rupture and eventually cell death. Group 17 Elements.
General introduction electronic configuration oxidation states occurrence trends in physical and chemical properties. Effects of KMnO4 treatment on the flexural impact and thermal properties of sugar palm fiber-reinforced thermoplastic polyurethane composites. JOM 70 2018 pp.
CrossRef View Record in Scopus Google Scholar. Mechanical and thermal properties of sugar palm fiber reinforced thermoplastic. The mechanical physical and chemical properties of aluminum foil such as its barrier effect deadfold properties and suitability for food contact enable a wide range of applications in many different products and sectors Lamberti and Escher 2007.
The material is light but strong can be formed and converted into complex shapes has a high thermal and electrical conductivity and. Alkanes- Nomenclature isomerism conformations ethane only physical properties chemical reactions including free radical mechanism of halogenation combustion and pyrolysis. Alkanes-Nomenclature structure of double bond ethene geometrical isomerism physical properties methods of preparation.
Addition of hydrogen halogen water hydrogen halides Markovnikovs. Nature of physical laws. Physics technology and society.
Motion in a straight line. Frame of reference Motion in a straight line. Position-time graph speed and velocity.
Intuitive concept of force Inertia Newtons first law of motion. Momentum and Newtons second law of motion. Definition Properties Examples 3.
What is the Difference Between Primary and Secondary Standard Solution Comparison of Key Differences. Hygroscopic Primary Standard Reference Material Secondary Standard Solvent Standardization. What is a Primary Standard Solution.
Primary standard solutions are solutions made out of primary standard substances. Activated carbon has a high capacity for BTEX compounds. Carbon uses strong physical adsorption properties to adsorb these organic compounds.
Hydrosils HS-AC is a granular activated carbon GAC manufactured with high surface area fine pore structure and superior hardness. Hydrosil provides coconut shell activated carbon coal based. Concepts covered in Elements of Groups 16 17 and 18 are Anomalous Behaviour of Fluorine Anomalous Behaviour of Oxygen Atomic and Physical Properties of Elements of Group 16 17 and 18 Chemical Properties of Elements of Groups 16 17 and 18 Chlorine Concept of Group 18 Elements Electronic Configuration of Elements of Group 16 17 and 18 Occurrence of Elements of Groups 16 17 and 18.
When it comes to the elements around us we can observe a variety of physical properties that these elements display. The study of hybridization and how it allows the combination of various molecules in an interesting way is a very important study in science. Understanding the properties of hybridisation lets us dive into the realms of science in a way that is hard to grasp in one go but.
Properties of Transition Metals. KMnO4 is potassium permanganate where manganese is in the 7 state with no electrons in the 4s and 3d orbitals. CeAr 4s0 3d0nonumber Since the 3p orbitals are all paired this complex is diamagnetic.
Oxidation states of transition metals follow the general rules for most other ions except for the fact that the d. Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table a highly reactive nonmetal and an oxidizing agent that readily forms oxides with most elements as well as with other compoundsOxygen is Earths most abundant element and after hydrogen and helium it is the third-most abundant element in the universe.
Physical Education Syllabus. Political Science Syllabus. Exams Prep Master Updated On - Jul 26 2021.
CBSE Class 12 Chemistry is divided into two sections. The Theory paper consists of a total of 70 marks while the Practical section carries 30 marks in total. Acetone CH3COCH3 also known as 2-propanone and dimethylketone is a colorless volatileflammable liquid that boils at 56C 133 OF.
It is misciblewith water and is oftenused as a solventin the manufacture of lacquers and paints. Clear colorless liquid with a sweet fragrant odor. Odor threshold concentrations ranged from 42 ppm v.
Colorless hygroscopic volatile liquid with a sweet pungent odor. Odor threshold concentration is 330 ppb quoted Keith and Walters 1992. Ether was supposedly discovered by Raymundus Lullus 12321315 around 1275 although there is no extant evidence of this in his writings.
The discoverer of ether is often credited to the German physician and botanist. Dielectric Properties Reference for tensor and properties. KMnO4 and Na2SO4 Aldrich were used as starting materials for the electrosynthesis of MnO2.
Commercial Ni plaques with 80 volume porosity and thickness of 18mm Vale Company were used as substrat 101002chem201203553. Synthesis and morphological control. According to the synthetic process.