
Flammable solids self-reactive substances solid desensitised explosives and polymerizing substances. Flammable solids self-reactive substances solid desensitised explosives and polymerizing substances.
Fire Hazards in Presence of Various Substances.
Is sodium hypochlorite flammable. Sodium hypochlorite commonly known in a dilute solution as bleach is a chemical compound with the formula NaOCl or NaClO comprising a sodium cation Na and a hypochlorite anion OCl or ClO. It may also be viewed as the sodium salt of hypochlorous acid. The anhydrous compound is unstable and may decompose explosively.
It can be crystallized as a pentahydrate NaOCl 5 H 2 O a. Sodium hypochlorite is a strong oxidator and reacts with flammable compounds and reductors. Sodium hypochlorite solution is a weak base that is inflammable.
These characteristics must be kept in mind during transport storage and use of sodium hypochlorite. What happens to the pH value when sodium hypochlorite is added to water. Due to the presence of caustic soda in sodium hypo chlorite the.
Soln of sodium hypochlorite containing 045-050 g of the salt in 100 ml. Prepd by diluting with distilled water a soln of sodium hypochlorite adding a 5 soln of sodium bicarbonate nad adjusting to proper strength and concn according to procedure described in NF. May be prepared also from 154 g chlorinated lime 30 available chlorine 77 g anhydrous sodium carbonate and 64 g sodium.
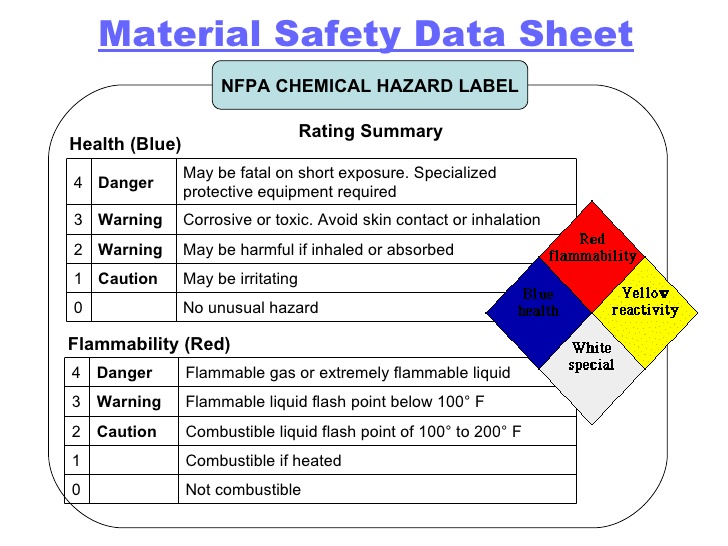
Sodium Hypochlorite Section 1. Chemical Product and Company Identification Product Name. Flammability of the Product.
Fire Hazards in Presence of Various Substances. Combustible materials metals organic. Containers may explode when heated.
Releases chlorine gas when heated above 35ºC. Anhydrous sodium hypochlorite is very explosive. Hypochlorites react with urea to form nitrogen trichloride which explodes.
Explosive reaction with formic acid at 55ºC phenylacetonitrile. Reacts to form explosive products with amines ammonium salts aziridine and methanol. This product is not flammable.
Use fire-extinguishing media appropriate for surrounding materials. 9th August 2007 Sodium Hypochlorite SPECIFIC HAZARDS Chlorine. PROTECTIVE MEASURES IN FIRE Self contained breathing apparatus and full protective clothing must be worn in case of fire.
6 ACCIDENTAL RELEASE MEASURES PERSONAL PRECAUTIONS Wear protective. Sodium hypochlorite disintegrates when heated or if it contacts acids sunlight certain metals and poisonous and corrosive gases including chlorine gas. It is a strong oxidant that reacts with flammable compounds and reducing agents and it is flammable.
These characteristics must be kept in mind during transport storage and use. The presence of caustic soda in sodium hypochlorite means. Sodium Hypochlorite 1 ppm 1 ppm Sodium Hydroxide 2 mgm3 2 mgm3.
Good general ventilation typically 10 air changes per hour should be used. Ventilation rates should be matched to conditions. If applicable use process enclosures local.
Sodium is a chemical element with the symbol Na from Latin natrium and atomic number 11. It is a soft silvery-white highly reactive metal. Sodium is an alkali metal being in group 1 of the periodic table.
Its only stable isotope is 23 Na. The free metal does not occur in nature and must be prepared from compounds. Sodium is the sixth most abundant element in the Earths crust and exists.
Sodium hypochlorite 7681-52-9 5 - 10 The exact percentage concentration of composition has been withheld as a trade secret. FIRST AID MEASURES First aid measures General Advice Call a poison control center or doctor immediately for treatment advice. Show this safety data sheet to the doctor in attendance.
Eye Contact Hold eye open and rinse slowly and gently with water for 15 - 20. Sodium potassium powdered aluminum Carbon dioxide carbon tetrachloride and other chlorinated hydrocarbons water Bromine chlorine fluorine and iodine. Do not use CO2 water or dry chemical extinguishers.
Use Class D extinguisher eg Met-L-X or dry sand. Aluminum and its Alloys especially powders Acid or alkaline solutions ammonium persulfate and water chlorates chlorinated. Since alcohol is flammable limit its use as a surface disinfectant to small surface-areas and use it in well-ventilated spaces only.
Prolonged and repeated use of alcohol as a disinfectant can also cause discoloration swelling hardening and cracking of rubber and certain plastics. Bleach is a strong and effective disinfectant its active ingredient sodium hypochlorite is. Sodium hydroxide is also used to produce sodium hypochlorite a water disinfectant.
Sodium Hydroxide in Food Production. Sodium hydroxide is used in several food processing applications such as curing foods like olives or helping to brown Bavarian-style pretzels giving them their characteristic crunch. Sodium hydroxide is used to remove skins from tomatoes potatoes and other fruits and.
Caustic soda Lye Sodium hydroxide Soda lye Sodium hydrate CAS No. DOT ID Guide. 1823 154dry solid 1824 154solution Formula.
Sodium Hypochlorite Ethyl Acetate Iodine Benzoyl Peroxide Potassium Dichromate Chlorates Bromates and Superoxides Ammonium Persulfate Ferric chloride Store in secondary containment Combustibles Fire Hazard separately from combustibles and Flammables Gas Generation flammable materials Organic Materials Toxic Gas Reducing Agents. Some are oxidizers and may ignite combustibles wood paper oil clothing etc. Contact with metals may evolve flammable hydrogen gas.
Containers may explode when heated. For electric vehicles or equipment ERG Guide 147 lithium ion batteries or ERG Guide 138 sodium batteries should also be consulted. Substances liable to spontaneous combustion.
Substances which on contact with water emit flammable gases. Flammable solids self-reactive substances solid desensitised explosives and polymerizing substances. We will be publishing Issue 1 of The School STEM Technician at noon on the 1st December 2021 via the SSERC website.
This is our newest publication and has been created to support the school technician profession in Scotland. DS WCSS104 Flammable Liquid sign DS WCSS105 Combustible Liquid sign DS WCSS106 Multi Calcium Hypochlorite sign DS WCSS107 Flammable Gas sign DS WCSS108 Multi Sodium Hypochlorite sign DS WCSS109 Multi Sodium Hydroxide Caustic Soda sign. DS WCSS110 Multi Sodium Metabisulphite sign DS WCSS111 Multi Sulfuric Acid sign DS WCSS112 Multi Ferric.
Titanium trichloride tert-Butyl hypochlorite Lithium diethylamide Lithium diisopropylamide Sodium methoxide Sodium methylate Sodium sulfide anhydrous or. Flammable liquids are defined by dangerous goods regulations as liquids mixtures of liquids or liquids containing solids in solution or suspension which give off a flammable vapour have a flash point at temperatures of not more than 60-65C liquids offered for transport at temperatures at or above their flash point or substances transported at elevated temperatures in a liquid state and. Bleach- Sodium Hypochlorite 525 bleach concentrate Phenols Quaternary Ammonium Compounds Accelerated Hydrogen Peroxide hydrogen peroxide anionic surfactants Botanicals Example- Benefect Thymol Silver Dihydrogen Citrate Example - PureGreen 24 unpleasant odor.
Must be stored separately from ammonia and flammable products21. Sodium nitrite and sodium thiosulfate. Other common examples of incompatible chemicals.
Chemical Is Incompatible and Should Not Be Mixed or Stored With. Chromic acid nitric acid hydroxyl compounds ethylene glycol perchloric acid peroxides permanganates. Chlorine bromine copper fluorine silver mercury.
Concentrated nitric and. Sodium chloride also called brine into sodium hydroxide chlorine and hydrogen gas. For hypochlorite sweetening a treatment step in the removal of various sulfur compounds.
Soda Ash Replacement - Caustic soda can be used interchangeably for many applications in glass paper pulp phosphates and silicates industries. Renewable Fuels - Caustic soda is used for pH adjustment and formation. When hydrogen peroxide comes in contact with flammable substances such as wood paper oil or cotton cellulose spontaneous ignition may occur.
When hydrogen peroxide is mixed with organic matter such as alcohols acetone and other ketones aldehydes and glycerol heavy explosions may occur. When hydrogen peroxide comes in contact with substances such as iron copper chromium.