
Hydrochloric acid is a clear highly corrosive strong acid. This is a viscous solution that is colorless odorless and non-volatile.
May react with active metals including such structural metals as aluminum and iron to release hydrogen a flammable gas.
Is phosphoric acid a strong acid. Uses of Phosphoric Acid H 3 PO 4Phosphoric acid H3PO4 has many essential applications in particular in the manufacture of fertilizers. Many acids are derived from phosphate rocks by a wet process based on the reaction between phosphate rocks and acid solutions1 This acid H3PO4 is a medium-strong acid but is also highly corrosive to ferrous or ferrous alloys. Phosphoric acid is commonly encountered in chemical laboratories as an 85 aqueous solution which is a colourless odourless and non-volatile syrupy liquid.
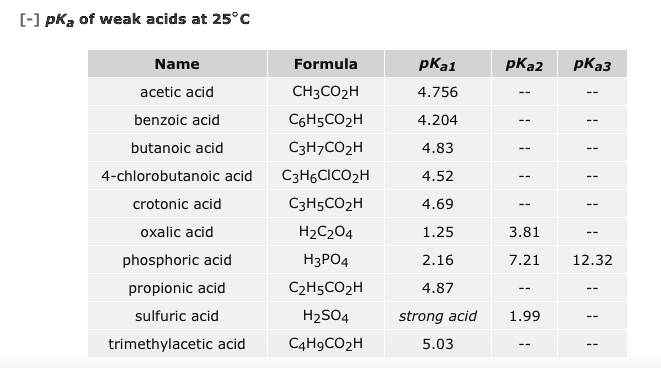
Although phosphoric acid does not meet the strict definition of a strong acid the 85 solution can still severely irritate the skin and damage the eyes. Titration of the phosphoric acid H 3 PO 4 is an interesting case. Although often listed together with strong mineral acids hydrochloric nitric and sulfuric phosphoric acid is relatively weak with pK a1 215 pK a2 720 and pK a3 1235.
That means titration curve contains only two inflection points and phosphoric acid can be titrated either as a monoprotic acid or as a diprotic acid. Phosphoric acid is used for metal cleaning and refining as well as fertilizer manufacturing. Its also found in disinfectants and detergents.
Its also found in disinfectants and detergents. With those usages its not so surprising that this acid is commonly found on lists of strong acids or chemicals commonly used in industry that can cause chemical burns. Is phosphoric acid strong or weak.
Phosphoric acid commonly comes in an 85 aqueous solution. This is a viscous solution that is colorless odorless and non-volatile. Its viscosity is similar to that of a syrup but is still pourable.
It is a weak acid. The strength of an acid is determined by several factors namely. Strong acids have higher electronegativity in their.
PHOSPHORIC ACID reacts exothermically with bases. May react with active metals including such structural metals as aluminum and iron to release hydrogen a flammable gas. Can initiate the polymerization of certain classes of organic compounds.
Reacts with cyanide compounds to release gaseous hydrogen cyanide. May generate flammable andor toxic gases in contact with. Phosphoric acid also referred to as phosphoricV acid or orthophosphoric acid is one of the popular and most used acids.
As such the raw form of this acid is extracted from phosphate rocks whereas more pure form is produced industrially from white phosphorus. Pure phosphoric acid is usually in a crystalline solid-state and in less concentrated form. Generally it is a colourless syrupy.
To check If Nitric acidHNO 3 a strong or weak first we have to take a clear understanding of the differences between strong and weak acids. A strong acid is generally a compound that dissociates completely or is 100 ionized in a solution to yield H ions which means no undissociated acid remains in the solution all moles of acid completely break off and release a lot of H ions. Phosphoric acid market continues to strengthen on September 18.
The violent rise of raw materials drives the price of phosphoric acid to soar. The price of phosphoric acid is tens of thousands of yuan 96-910 due to tight supply. The market for phosphoric acid continues to rise 830-96 The phosphoric acid market once again set sail in August.
Page 1 of 8 MSDS -Phosphoric acid 85 Material Safety Data Sheet MSDS - Phosphoric acid 85 1Chemical Product and Company Identification Product Name. Phosphoric acid 85 Catalog Codes. SLP5569 SLP4555 SLP1732 CAS.
Acid strength is the tendency of an acid symbolised by the chemical formula to dissociate into a proton and an anion The dissociation of a strong acid in solution is effectively complete except in its most concentrated solutions. Examples of strong acids are hydrochloric acid perchloric acid nitric acid and sulfuric acid. A weak acid is only partially dissociated with.
Acid strength refers to the tendency of a specific acid to dissociate into a proton and an anionStrong acids completely dissociate and the chemical formula for this process is HA H AOn the other hand weak acids only partially dissociate with the formula being HA H A. In terms of rust removal these two types of acid react. Phosphoric acid is made from the mineral phosphorus which is found naturally in the body.
It works with calcium to form strong bones and teeth. It also helps support kidney function and the way. Selenic acid - a strong acid H2SeO4 analogous to sulfuric acid.
Sulfonic acid sulphonic acid - an acid derived from sulphuric acid. Titanic acid - a white weak acid that is a hydrated form of titanium dioxide. Perchloric acid - a powerful oxidizing agent.
Carboxylic acid - an organic acid characterized by one or more carboxyl groups. Aminobenzoic acid - a derivative of. Sodium acid pyrophosphate can be used as a leavening chemical for bread to help it rise.
Its used in sausage to enhance flavor and color. In french fries the chemical reduces levels of a carcinogen called acrylamide according to an article from the Center for Science in the Public Interest. It also prevents discoloration in potatoes and sugar syrups.
In canned tuna it prevents harmless. An unprecedented sequential CH olefinationasymmetric 41 spirocyclization under a simple CoIIchiral spiro phosphoric acid SPA binary system is reported. The strong acids are hydrochloric acid nitric acid sulfuric acid hydrobromic acid hydroiodic acid perchloric acid and chloric acid.
The only weak acid formed by the reaction between hydrogen and a halogen is hydrofluoric acid HF. While technically a weak acid hydrofluoric acid is extremely powerful and highly corrosive. Strong acids dissociate completely into their.
Gujarat Alkalies and Chemicals Limited has announced that its product ie. Ortho Phosphoric Acid has been granted License by Bureau of Indian. Hydrochloric acid is a clear highly corrosive strong acid.
Its found in diluted form as muriatic acid. The chemical has many industrial and lab uses. Muriatic acid for industrial purposes typically is 20 to 35 percent hydrochloric acid while muriatic acid for household purposes ranges between 10 and 12 percent hydrochloric acid.
HCl is the acid found in gastric juice. The acid ionization represents the fraction of the original acid that has been ionized in solution. Therefore the numerical value of K a is a reflection of the strength of the acid.
Weak acids with relatively higher K a values are stronger than acids with relatively lower K a values. Because strong acids are essentially 100 ionized the concentration of the acid in the denominator is nearly. Phosphoric AcidACS Created by Global Safety Management Inc.
Identification of the substancemixture and of the supplier Product name. Phosphoric AcidACS ManufacturerSupplier Trade name. S25470B Recommended uses of the product and uses restrictions on use.
The ones that are weaker than oxalic acid include the citric phosphoric and acetic acids. All three of them can remove rust effectively which is why people often recommend them over other products. However they are slow-acting and need more time to remove everything.
On the other hand there are strong solutions such as sulfuric and hydrochloric acids which remove rust much faster than. For Pros and DIYers Klean-Strip is the leading brand of solvents thinners removers and cleaners that are dependable for reliable results. Klean-Strip Concrete Metal Prep does the job of three products.
1 Removes rust from iron and steel surfaces 2 treats metal surfaces including galvanized and aluminum to allow better paint adhesion and 3 etches concrete to allow paint or. Sulfamic acid is the safest to handle and recommended for non-professionals. Phosphoric acid creates less fumes.
Use it in rooms that contain stainless steel or other acid-vulnerable metals. Its also a good choice if youre just cleaning off mineral deposits. Muriatic acid hydrochloric acid is the most dangerous option and produces strong.
Citric acid occurs naturally in some foods particularly fruit and it is a common additive in certain foods and beverages. It serves various purposes as an additive explains the US. Food and Drug Administration.
Citric acid functions as a preservative combating food spoilage and degradation. It also helps regulate the acidity or pH of food products and adds a sour or acidic taste to.