It is a type of potential energy which is obtained from conservative Coulomb forces. It is a type of potential energy which is obtained from conservative Coulomb forces.

Explosives 135-trinitrotoluene nitroglycerin etc Refrigerant liquid N 2 Anesthetic laughing gas Comprises about 15 of proteins by weight.
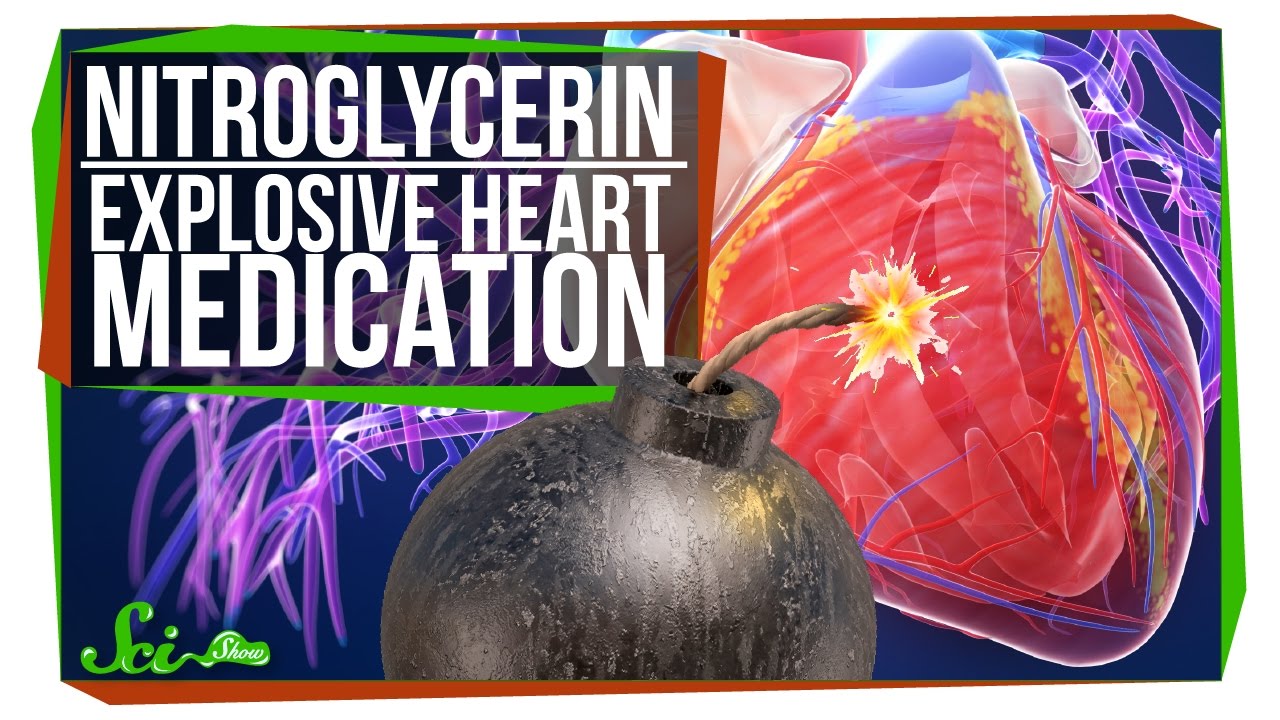
Is nitroglycerin flammable. Nitroglycerin is a nitroglycerol that is glycerol in which the hydrogen atoms of all three hydroxy groups are replaced by nitro groups. It acts as a prodrug releasing nitric oxide to open blood vessels and so alleviate heart pain. It has a role as a vasodilator agent a nitric oxide donor an explosive a prodrug a tocolytic agent a muscle relaxant and a xenobiotic.
Nitroglycerin distributes widely throughout the body tissues and is approximately 60 plasma protein-bound. The metabolites of nitroglycerin 13- and 12-glyceryl dinitrate are much less potent than the parent compound and have a half-life of approximately 40 minutes compared to a parent half-life of 1 to 3 minutes. The metabolites are.
Nitroglycerin mixture desensitized solid nos with more than 2 but not more than 10 nitroglycerin by mass UN 3320. Sodium borohydride and sodium hydroxide solution with not more than 12 sodium borohydride and not more than 20 sodium hydroxide by mass UN 3321. Radioactive material low specific activity LSA-II non-fissile or fissile excepted.
Nitroglycerin solution in alcohol with more than 1 percent but not more than 5 percent nitroglycerin UN 3065. Paint or Paint related material UN 3067 to 3069. No longer in use UN 3070.
Ethylene oxide and dichlorodifluoromethane mixture with not more than 125 percent ethylene oxide UN 3071. Mercaptans liquid toxic flammable nos. But while it is comparatively simple to use gasoline is of course highly flammable and must be handled with care.
Many propellants are highly toxic to a greater degree even than most war gases. Some are so corrosive that only a few special substances can be used to contain them. Some may burn spontaneously upon contact with air or upon contacting any organic substance or in certain cases.
I Contains only the residue of a Division 21 flammable gas or Class 3 flammable liquid material. For liquids the meter prover must be drained to not exceed 10 of its capacity or to the extent that draining of the meter prover is impracticable to the maximum extent practicable. For gases the meter prover must not exceed 25 of the marked pressure rating.
Nitrocellulose block wet with not less than 25 alcohol appears as a white solid. A mixture of the dinitrates and trinitrate of cellulose and ethanolExposure to heat may evaporate the solvent leaving a residue that is subject to self-accelerating decomposition and may explode if. Flammableexplosiveaerosol containersvolatile liquids Examples.
Benzocaine spray collodion m edical or anesthetic gases for surgery nitroglycerin spray. Medications that are difficult to replace andor pose confidentiality issues. Patients Own Medications EmployeeOutpatient prescriptions Research or Investigational Medications Expensive items Medications.
Nitric acid is used in the production of ammonium nitrate plastics dyes and explosives such as nitroglycerin and TNT. When nitric acid is combined with HCL a fuming liquid known as aqua regia is formed capable of dissolving gold and platinum. Nitric acid is also utilised in the medical industry to remove warts and as a colorimetric test to distinguish drugs.
When exposed to the skin. Instructed to take nitroglycerin at the first sign of angina to rest in a lying or sitting position. Patient was instructed on measures for fire safety.
Store flammable substances in a well-ventilated. Patient was instructed on skin care. Keep the skin clean and dry.
When bathing or showering use war. Instructed on some signssymptoms of respiratory infection such as. Nitroglycerin is a volatile vasodilator frequently used in treating or preventing attacks of chest pain.
It widens blood vessels making it easier for the heart to pump. However overdosing on the chemical can lead to fever confusion headache dizziness vomiting trouble breathing vision problems bloody diarrhea fainting cold skin increased heart rate seizures and death. Nitroglycerin Water Reactive when it contacts water it produces gases that are flammable or a health hazard.
Metallic sodium Carcinogen can cause cancer. Benzene Ethylene Oxide Formaldehyde Corrosive cause irreversible damage to the skin eyes or the respiratory tract at the site of contact. Acids and Bases Irritant can cause.
All smokeless powders are extremely flammable. By design they are intended to burn rapidly and vigorously when ignited. Oxygen from the air is not necessary for the combustion of smokeless powders since they contain sufficient built-in oxygen to burn completely even in an enclosed space such as the chamber of a firearm.
Ignition occurs when the powder granules are heated. If a vehicle is powered by a flammable liquid and a flammable gas internal combustion engine it must be consigned under the entry Vehicle flammable gas powered These entries include hybrid electric vehicles powered by both an internal combustion engine and wet sodium or lithium batteries installed. If a fuel cell engine is installed in a vehicle the vehicle must be consigned using.
Place the patch on a hairless area of skin chest back or abdomen and rotate sites. Remove old patch wash skin with soap and water and dry thoroughly before applying new patch. Remove the patch at night to reduce the risk of developing tolerance to nitroglycerin.
Nitroglycerin CAS No 55-63-0 7 Antimony CAS No 7440-36-0 3 Nickel CAS No 7440-02-0 1 Zinc oxide CAS No 1314-13-2 025 Graphite CAS No 7782-42-5 025 The hazardous components of this product are encased within a shell and are unlikely to be released under normal handling conditions. Therefore the health and environmental hazards associated with nitrocellulose and. STONE Its heavy but breakable.
LAVA It burns everything. ANT When they touch solids they create a mysterious path. TORCH Burn everything but weak in water.
And burn the trees. SOAPY Soapy water makes bubbles. The common solvent acetone flammable and transparent with a characteristic odor.
Acetylene C 2 H 2. Also called ethyne it is an alkyne gas lighter than air and colorless very flammable. Ethyl ethanoate CH 3 -COO-C 2 H 5.
Also known as ethyl acetate or vinegar ether used as a solvent. Formol CH 2 0. Used as a biological material.
Its heavy but breakable. When they touch solids they create a mysterious path. TORCH Torch burns everything but keep water away.
SOAPY Soapy water makes bubbles. All smokeless powders are extremely flammable. By design they are intended to burn rapidly and vigorously when ignited.
Oxygen from the air is not necessary for the combustion of smokeless powders since they contain sufficient built-in oxygen to burn completely even in an enclosed space such as the chamber of a firearm. Ignition occurs when the powder granules are heated. However the unstable nature of nitroglycerin is greatly controlled when mixed with diatomaceous earth.
Thus dynamites prepared in this manner only explode when ignited releasing large amounts of heat nitrogen and other highly flammable gases. It is a type of potential energy which is obtained from conservative Coulomb forces. It is associated with the.
The cells contain no flammable material and have a protective fluoride coating for the same reason. Cost of 1 gram 1 mole. Explosives 135-trinitrotoluene nitroglycerin etc Refrigerant liquid N 2 Anesthetic laughing gas Comprises about 15 of proteins by weight.
Scheele independently in 1773-74. It is colorless yet toxic and flammable gas with rotten eggs-like smells. This gas can be formed due to biological activity when bacteria breaks down organic substances in non-oxygen state or it is commonly called as anaerobic activity.
Examples of hydrogen sulfide can be found as in sewerage channels. Hydrogen sulfide is heavier than air that it is more likely to collect and settle in a. From both nitrocellulose and nitroglycerin.
All smokeless powders are extremely flammable. By design they are intended to burn rapidly and vigorously when ignited. Oxygen from the air is not necessary for the combustion of smoke- less powders since they contain sufficient built-in oxygen to burn completely even in an enclosed space such as the chamber of a firearm.
Ignition occurs when the.