
Or adjacent members of homologues series. CCl 4 and SiCl 4.
To circumvent the problem green.
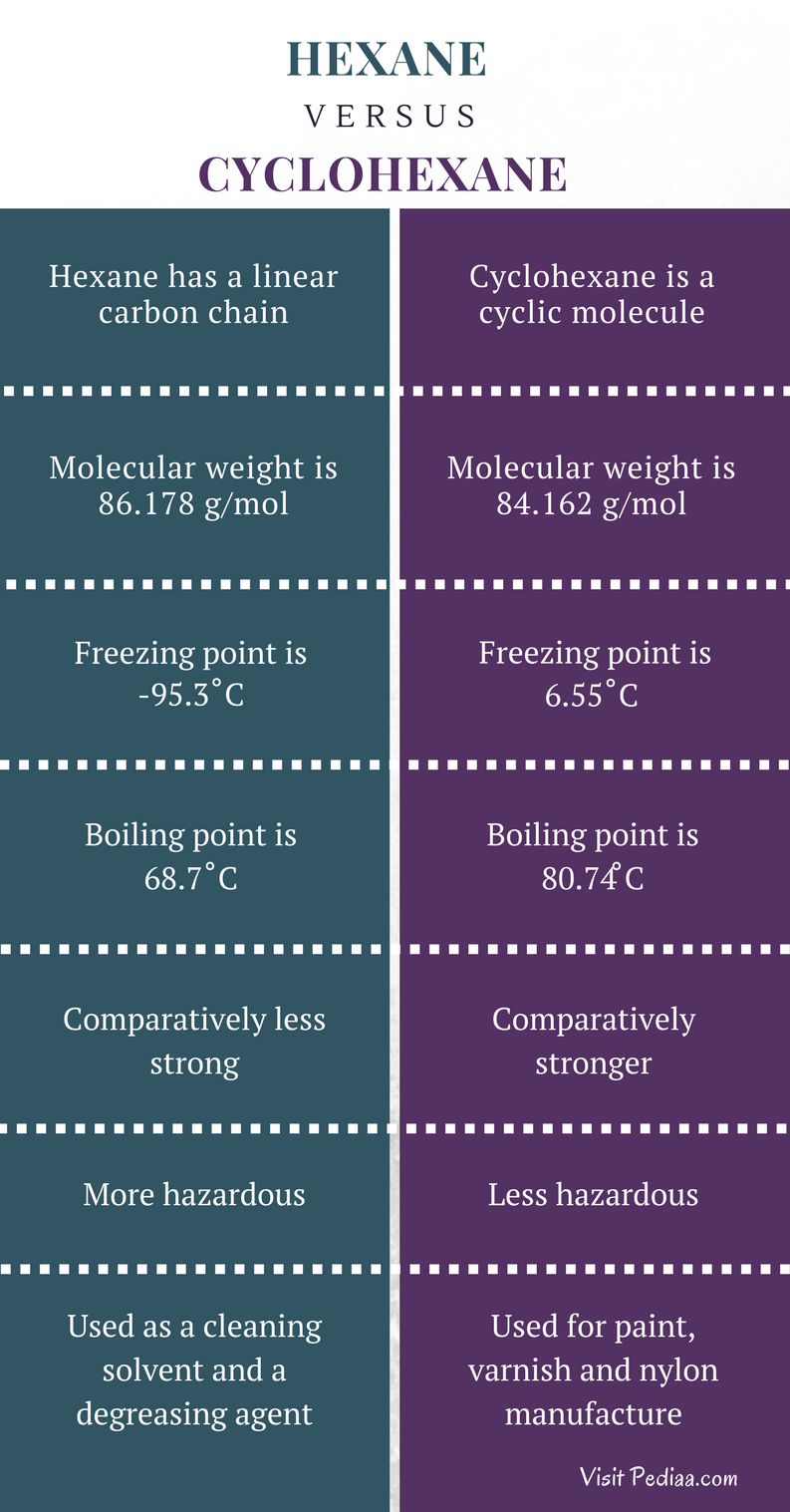
Is n hexane the same as hexane. 480 480 480 480 480 480 480 indicates greater than. Having a normalized breakthrough time of 10 minutes or less. Special Warnings from DuPont.
Serged and bound seams are degraded by some hazardous liquid chemicals such as strong acids and should not be worn when these chemicals are present. Pure n-Hexane is a colorless liquid with a slightly disagreeable odor. It is highly flammable and its vapors can be explosive.
Puren-Hexane is used in laboratories. Most of then-Hexane used in industry is mixed with similar chemicals called solvents. The major use for solvents containing n-Hexane is to extract vegetable oils from crops such as soybeans.
These solvents are also used as. CID 8857 Ethyl acetate CID 8058 Hexane Dates. 1 Structures Expand this section.
2 Names and Identifiers Expand this section. 3 Chemical and Physical Properties Expand this section. 4 Related Records Expand this.
In solvent extraction n-hexane is used as a solvent for its attributes such as simple recovery non-polar nature low latent heat of vaporization 330 kJkg and high selectivity to solvents. However usage of hexane as a solvent has lead to several repercussions such as air pollution toxicity and harmfulness that prompted to look for alternative options. To circumvent the problem green.
Normal-phase HPLC NP-HPLC which is not the most popular form of HPLC nowadays utilizes a polar stationary phase usually silica and less polar nonaqueous eluting solvents eg n-hexane and ethyl acetate mobile phase. The separation is based on the analytes ability to engage in polar interactions eg hydrogen bonding or dipoledipole type interactions with the sorbent surface. N-Hexane n- Hexane is a neurotoxin which has been responsible for the poisoning of several workers in Chinese electronics factories in recent years.
110 111 112 113. Turmeric extracts prepared in n-hexane water chloroform and ethanol were applied to meals as preservatives and antibacterial agent. The samples were assessed microbiologically total bacterial total fungal and total coliform counts and organoleptically color odor taste at day zero and after 15 days intervals.
Meals autoclaved for shorter time 5 min and treated with combination of 1. For n-hexane 50 ppm. Therefore the TLV of the mixture is TLVmix 1 00505 09550 84 ppm corresponding to 80 ppm hexane and 04 ppm benzene.
For an instrument calibrated on isobutylene the reading corrsponding to the TLV is. A common practice is to set the lower alarm limit to half the TLV and the higher limit to the TLV. Thus one would set the alarms to 13 and 26 ppm.
Among the hydrocarbons aromatic hydrocarbons have higher SG lower API than paraffinic hydrocarbons with the same number of carbon atoms. For example benzene has an SG of 0883 API of 287 whereas n-hexane has an SG of 0665 API of 813. Therefore the heavy high-density crude oils tend to have high concentrations of aromatic hydrocarbons whereas the light low-density crude.
N-hexane CH 3 t 088 088 086 089 089 090 CH 2 m 126 128 125 124 128 129 HMPAg CH 3 d95 265 259 253 240 257 264 261 methanol CH 3 sh 349 331 316 307 328 334 334 OH sch 109 312 401 216 nitromethane CH 3 s 433 443 442 294 431 434 440 n-pentane CH 3 t7 088 088 086 087 089 090 CH 2 m 127 127 127 123. Normally such a series progresses from non-polar solvents such as n-hexane to polar solvents such as methanol or water. And the time at which the solute elutes.
In the same way the elution volume is the volume of eluent required to cause elution. Under standard conditions for a known mix of solutes in a certain technique the elution volume may be enough information to identify solutes. NMR Chemical Shifts of Common Laboratory Solvents as Trace Impurities Hugo E.
Gottlieb Vadim Kotlyar and Abraham Nudelman Department of Chemistry Bar-Ilan University. Proximately the same level as a typical sample. At each temperature the D 2O samples were left to equilibrate for at least 10 min before the data were collected.
In order to avoid having to obtain hundreds of spectra we prepared seven stock solutions containing approximately equal amounts of several of our entries chosen in such a way as to prevent intermolecular interactions and possible. C have the same molecular formula. D have a different content of the isotopes of hydrogen.
E react vigorously with one another. Which of the following hydrocarbons does not have isomers. A C 7 H 16 b C 6 H 14 c C 5 H 10 d C 4 H 8 e C 3 H 8.
The name of the alkane isomer of cis-3-hexene is. A 2-methylpentane b 3. Too different in size and are of the same chemical nature.
Mixture of isomers such as ortho- meta- para- xylene. Or adjacent members of homologues series. I 12N 101 y.
I Vapor-phase mole fraction. I Liquid-phase mole fraction. I Vapor.
N - Hexane. Home Login Join Free. Magazine for Price Alerts Get regular chemical price alerts through Email and mobile SMS.
Get daily chemical price alerts via SMS and email. Get intra-day chemical price alerts for major price fluctuations. Get chemical prices at major Indian.
N-Hexane 2-Methylpentane 3-Methylpentane 23-Dimethylbutane 22 - Dimethylbutane Based upon the number of carbon atoms attached to a carbon atom the carbon atom is termed as primary 1 secondary 2 tertiary 3 or quaternary 4. Carbon atom attached to no other carbon atom as in methane or to only one carbon atom as in ethane is called primary carbon atom. A The double bond would have the same strength as the single bond.
B The double bond would be stronger than but less than twice as strong as the single bond. C The double bond would have exactly twice the strength of the single bond. D The double bond would have more than.
Hence C 5 H 12 is called pentane C 6 H 14 is called hexane C 7 H 16 is called heptane and so forth. Straight-chain alkanes are sometimes indicated by the prefix n-for normal to distinguish them from branched-chain alkanes having the same number of carbon atoms. Although this is not strictly necessary the usage is still common in cases where there is an important difference in properties.
On the other hand the chemical n-hexane is not deposited or accumulated in the body. It is broken down in the liver. One of the breakdown products can attack nerve cells in the fingers and toes.
These kinds of cell are not replaced by the body easily. With continued exposure for many years the damage to the nerve cells increases until a point is reached where symptoms appear in the fingers. On prolonged heating with HI it forms n-hexane suggesting that all the six carbon atoms are linked in a straight chain.
Glucose reacts with hydroxylamine to form an oxime and adds a molecule of hydrogen cyanide to give cyanohydrin. These reactions confirm the presence of a carbonyl group C O in glucose. Glucose gets oxidised to.
Mineral surface rather than to molecules of the same fluid. The interfacial forces give rise to what is known as a capillary pressure. N-Hexane-Air 00184 184 n-Octane-Air 00218 218 Water-Oil Approx.
350 Mercury-Air 03680 3680 Units. 1 Nm 1000 dynescm Rmean F p s 4 F 2R mean 2R inner 2R outer. Petrophysics MSc Course Notes Fluid Saturation and Capillary Pressure.
N-Hexane 110-54-3 C H 8618 687 1513 202 57 43 86 41 hexyl-hydride MN9275000 6 14 n-Heptane 142-82-5 C H 10021 984 458 61 43 71 57 MI7700000 10041 7 16 n-Octane 111-65-9 C H 11423 1257 140 19 43 85 114 57 RG8400000 8 18 n-Decane 124-18-5 C H 14229 174 14 02 43 57 71 41 decyl hydride HD6500000 142 10 22 Ketones. Below the explosive or flammable limit the mixture is too lean to burn. Above the upper explosive or flammable limit the mixture is too rich to burn.
The Auto-Ignition Temperature is not the same as Flash Point - The Flash Point indicates how easy a chemical may burn. This means that total volume of solution is exactly same as the sum of the volume of solute and solution. CCl 4 and SiCl 4.
Ethyl Bromide and Ethyl Iodide. N-Butyl Chloride and n-Butyl Bromide. The solutions which dont obey Raoults law at.