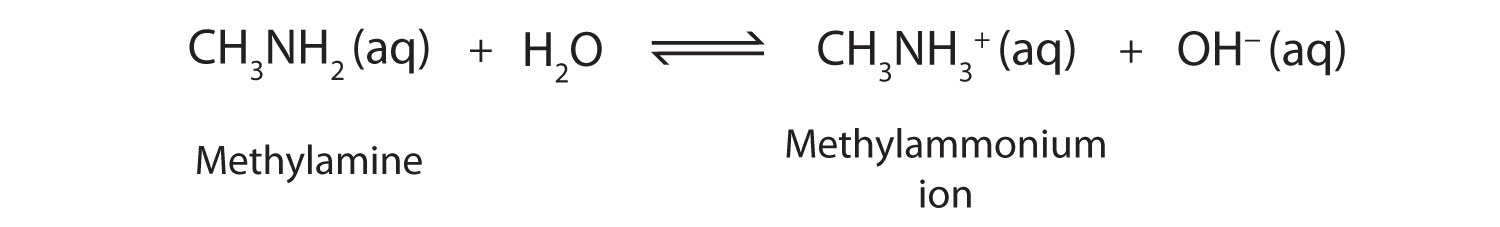
It is also known as methanamine MeNH2 methyl ammonia methyl amine and aminomethane. The position of equilibrium varies from base to base when a weak base reacts with water.
At equilibrium both the acid and the conjugate base are present in solution.
Is methylamine a strong base. A compound is a. So Is Methylamine CH 3 NH 2 a strong base or weak base. CH 3 NH 2 is considered a weak base because when it is dissolved in an aqueous solution then not all the molecules of it react with water to yield OH ions very few molecules of CH 3 NH 2 react with water molecule ions and produce OH ion in the solution.
And the amount of OH produced in an. Methylamine is sold as a solution in methanol ethanol tetrahydrofuran or water or as the anhydrous gas in pressurized metal containers. Industrially methylamine is transported in its anhydrous form in pressurized railcars and tank trailers.
It has a strong odor similar to fish. Methylamine is used as a building block for the synthesis of numerous other commercially available compounds. Compare the values of the base dissociation constant for ammonia and methylamine.
K b ammonia 18 10-5 smaller K b. NaOH is a strong base it completely dissociates to form OH-and Na Set up a RICE. Table as shown below to find the equilibrium concentration of each species in solution.
NaOH aq OH-aq Na aq Initial concentration. Mol L-1 010. To know whether CaOH 2 is a strong base or weak you must know the basic difference between a strong base or a weak base.
A compound is a strong base when it completely dissociates in an aqueous solution and liberates a large number of hydroxide ionsAll moles of the strong base dissociate into hydroxide ionOH and no part remains undissociated into the solution. RbOH - rubidium hydroxide. CsOH - cesium hydroxide.
CaOH 2 - calcium hydroxide. SrOH 2 - strontium hydroxide. BaOH 2 - barium hydroxide.
C5H5N pyridine Remember any base that dissolves in water is an alkali and must have a pH above 7. The bases listed above ending with hydroxide are. PH586 The net ionic equation for the titration in question is the following.
CH_3NH_2H-CH_3NH_3 This exercise will be solved suing two kinds of problems. Stoichiometry problem and equilibrium problem. At the equivalence point the number of mole of the acid added is equal to the number o fmole of base present.
BOH H 2 O B aq OH-aq Examples of strong acids and bases are given in the table below. In aqueous solution each of these essentially ionizes 100. A weak acid or a weak base only partially dissociates.
At equilibrium both the acid and the conjugate base are present in solution. Strong acid reacts with strong base to form an acidic solution pH 7. Weak acid reacts with strong base to form an acidic solution pH 7.
When a weak acid reacts with a weak base the equivalence point solution is alkalinebase if the base is strong and acidic if the acid is strong. If the two concentrations are the same the equivalent pH value is neutral. For example when using a strong acid and a weak base an indicator that changes at a low pH is needed such as methyl orange 31-44.
As titrations curves using a weak acid and a weak base are highly irregular indicators cannot be used accurately. Instead a pH meter is often used. The word titration comes from the French word tiltre originally meaning the proportion of.
Methylamine CH 3 NH 2. Ammonium hydroxide NH 4 OH. An example of a weak base is ammonia.
It does not contain hydroxide ions but it reacts with water to produce ammonium ions and hydroxide ions. The position of equilibrium varies from base to base when a weak base reacts with water. The further to the left it is the weaker the base.
When there is a hydrogen ion gradient. CH 5 N Methylamine is a weak Lewis base. It is also known as methanamine MeNH2 methyl ammonia methyl amine and aminomethane.
Methylamine is most commonly encountered in pure form as a colorless gas although its also found as a liquid in solution with ethanol methanol water or tetrahydrofuran THF. Methylamine is the simplest primary amine. A strong base is a base which ionizes completely in an aqueous solution.
The most common strong bases are soluble metal hydroxide compounds such as potassium hydroxide. Some metal hydroxides are not as strong simply because they are not as soluble. Calcium hydroxide is only slightly soluble in water but the portion that does dissolve also dissociates into ions.
Disclaimer - accuracy of the values shown especially for the strong acids is questionable. Depending on the source pK a for HCl is given as -3 -4 or even -7. These values are usually not measured but calculated from thermodynamical data and should not be treated too seriously.
I pK b -log K b ii just like pK a strength of base increases with decreasing pK b See Table 186 The bigger is K b the more OH-is generated. The smaller is pK b the more OH-is generated. Base formula K b pK b Diethylamine NHEt 2 86 x 10-4 307 Methylamine NH 2Me 44 x 10-4.
It is a tertiary amine and a member of methylamines. It is a conjugate base of a trimethylammonium. 1 Structures Expand this section.
2 Names and Identifiers Expand this section. 3 Chemical and Physical Properties Expand this section. 4 Spectral Information Expand this section.
5 Related Records Expand this section. 7 Food Additives and Ingredients Expand. Metabolism was limited with only 5 being demethylated to methylamine.
The remainder of the dose was excreted unchanged. Pharmacokinetic studies indicated rapid t12ab 8 min and extensive absorption bioavailability 82 from the gastrointestinal tract followed by widespread distribution and a fairly prompt excretion t12el 6-7 hr with a plasma clearance of 190 mLmin. The common name for this very simple amine is methylamine no separators between methyl and amine.
While the conjugate acid of a strong base like hydroxide ion is a weak acid like water. The concept of pK a has already been developed as a measure of the acidity of Bronsted acids and we will also see that a corresponding concept pK b can be used as a measure of the basicity of bases. At the bottom left of Figure 1652 are the common strong acids.
At the top right are the most common strong bases. Notice the inverse relationship between the strength of the parent acid and the strength of the conjugate base. Thus the conjugate base of a strong acid is a very weak base and the conjugate base of a very weak acid is a strong base.
A strong strong b weak strong c strong weak d weak weak e none of these 17. Consider the titration of 300 mL of 020 M nitrous acid by adding 00500 M aqueous ammonia to it. The pH at the equivalence point is _____.
This is the titration of a weak acid with a weak base a greater than 7 b equal to 7 c less than 7. Organic Chemistry Acid-Base Equilibrium Acid-Base Equilibrium Part 1. How to Use the pKa Table In this lesson I want to talk about the fundamentals of the acid-base equilibrium and how we use it within the scope of organic chemistry.
But before we go into the details of the acid-base equilibrium itself lets review what a base and what an acid is according to different definitions. The leveling effect applies to solutions of strong bases as well. In aqueous solution any base stronger than OH is leveled to the strength of OH because OH is the strongest base that can exist in equilibrium with water.
Salts such as K_2O NaOCH_3 sodium methoxide and NaNH_2 sodamide or sodium amide whose anions are the conjugate bases of species that would lie. Sodium hydroxide a chemical compound with the formula NaOH is known to be a strong base. This is because sodium hydroxide undergoes almost complete ionization when it is dissolved in water.
Weak bases are the basic substances that do not completely ionize in water. An example of a weak base is ammonia. When NH 3 is dissolved in water a part of it dissociates into ammonium cation and.
In the conjugate base of ethane the negative charge is borne by a carbon atom while on the conjugate base of methylamine and ethanol the negative charge is located on a nitrogen and an oxygen respectively. Remember that electronegativity also increases as we move from left to right along a row of the periodic table meaning that oxygen is the most electronegative of the three atoms and. The pKa of ammonia itself is 38 which measures the equilibrium constant for dissociation of NH 3 to give its conjugate base NH 2 - and H.
That means that 38 is the pK a H of the amide ion NH 2 which you may have encountered before as the strong base NaNH 2 used to deprotonate terminal alkynes pKa 25. For example with Regression minute concentrations of some acidic and basic components in acid rain samples titrated with strong base can be determined individually or grouped as follows. Strong acids H2SO4 HNO3 weak carboxylic acid formic acetic bicarbonate H2CO3HCO3-CO3 and ammonium ion NH4 NH3 FORNARO A.
GUTZ IGR Wet deposition and related atmospheric.