
It collects in low-lying and enclosed poorly-ventilated areas such as basements manholes sewer lines under-ground telephone vaults and manure pits. Hydrogen sulfide is a flammable gas.
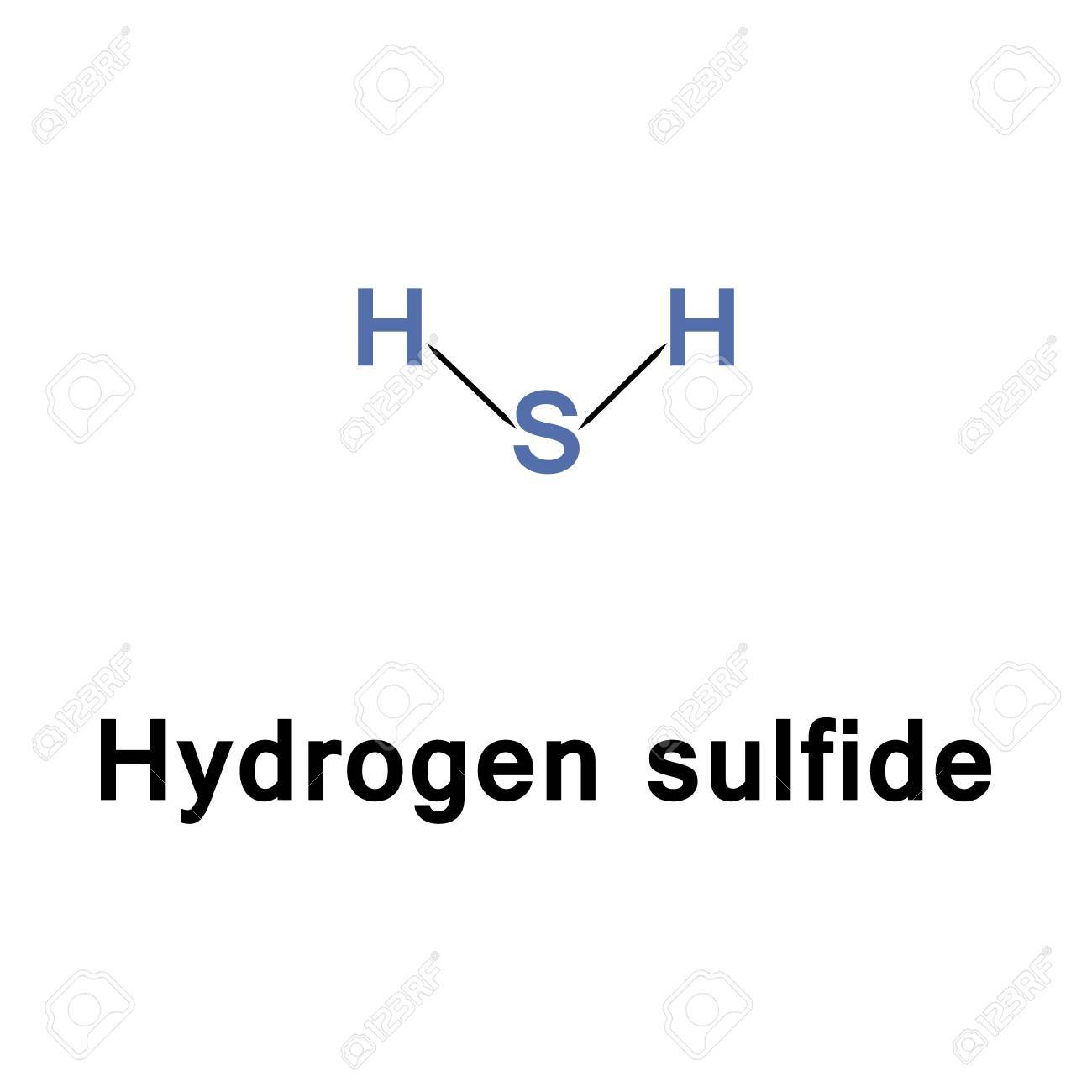
Because it is heavier than air hydrogen sulfide can collect in low-lying and enclosed spaces such as manholes sewers and underground telephone vaults.
Is hydrogen sulphide heavier than air. Hydrogen sulfide is slightly denser than air. A mixture of H 2 S and air can be explosive. Hydrogen sulfide burns in oxygen with a blue flame to form sulfur dioxide SO 2 and waterIn general hydrogen sulfide acts as a reducing agent especially in the presence of base which forms SH.
At high temperatures or in the presence of catalysts sulfur dioxide reacts with hydrogen. Hydrogen sulfide is slightly denser or heavier than air but fairly soluble in water. Hydrogen sulphide is a colourless poisonous gas that can lead to headaches even if it is inhaled in small quantities.
It has a distinct rotten egg smell. Hydrogen sulfide is a flammable gas. Chemical Properties of Hydrogen Sulphide.
A mixture of air and H 2 S can lead to an explosion. Oxygen and Hydrogen. Because it is heavier than air hydrogen sulfide can collect in low-lying and enclosed spaces such as manholes sewers and underground telephone vaults.
Its presence makes work in confined spaces potentially very dangerous. The health effects of hydrogen sulfide depend on how much H 2 S a worker breathes and for how long. However many effects are seen even at low concentrations.
Hydrogen sulfide is slightly heavier than air and may accumulate in enclosed poorly ventilated and low-lying areas. Children exposed to the same levels of hydrogen sulfide as adults may receive larger doses because they have greater lung surface areabody weight. Hydrogen sulfide is heavier than air and may travel along the ground.
It collects in low-lying and enclosed poorly-ventilated areas such as basements manholes sewer lines under-ground telephone vaults and manure pits. For work within confined spaces use appro-priate procedures for identifying hazards monitoring and entering confined spaces. The primary route of exposure is inhalation and.
Compound is heavier than air and may travel a considerable distance to source of ignition and flash back. It forms explosive mixtures with air over a wide range. Also reacts explosively with bromine pentafluoride chlorine trifluoride nitrogen triiodide nitrogen trichloride oxygen difluoride and phenyl diazonium chloride.
When heated to. Heavier than air H2S gas accumulates in low lying areas of poorly ventilated spaces. In oil and gas applications sour gas products containing H2S gas in the presence of air and moisture may form sulfuric acid capable of corroding metals.
Facility equipment including the internal surfaces of various components faces reduced durability and impact strength potentially leading to premature. One sulphur dioxide molecule reacts with two hydrogen sulphide molecules and produces three sulfur atoms and two water molecules. H 2 S properties.
SO 2 and H 2 S are very toxic gases and causes harmful injuries to humans animals and whole environment. Inhaling H 2 S in. Over more than 2500 km in the western USA where they carry 50 MtCO 2 yr-1 from natural sources to enhanced oil recovery projects in the west Texas and elsewhere.
The carbon dioxide stream ought preferably to be dry and free of hydrogen sulphide because corrosion is then minimal and it would be desirable to establish a minimum specification for pipeline quality carbon dioxide. Hydrogen sulphide generated in sewage and settled sludge is sealed within the static condition. Chlorine gas has specific weight that is 249 times heavier than air.
It is a yellowish green gas and is a strong irritant. Although its disinfecting effect is high its toxicity is also high. Operation and Maintenance 9 - 6 CHAPTER 9.
OCCUPATIONAL HEALTH HAZARDS AND. Solubility of hydrogen chloride fountain experiment. Setting of the apparatus procedure observation inference.
Method of preparation of hydrochloric acid by dissolving the gas in water- the special arrangement and the mechanism by which the back suction is avoided should be le arnt. Reaction with ammonia Acidic prope- rties of its solution reaction with. Iron oxide and hydrogen gas are formed by passing of steam over iron metal.
Both calcium Ca and magnesium Mg are heavier than water but still float over it. Both calcium and magnesium float over water surface because hydrogen gas is evolved when these metals react with water. It is in the form of bubbles which stick on the metal surface.
Flammable gases such as carbon monoxide and hydrogen sulphide are toxic at very low concentrations. Most vapours from flammable liquids are heavier than air and will accumulate near the ground. They can displace the air.
When there is not enough air or oxygen there is a hazard of asphyxiation suffocation. FEX002 Fire and Explosives 4 Revised May 2007 When flammable materials burn toxic. Metals below hydrogen are less reactive than hydrogen so cant displace hydrogen from acid.
Order of reactivityNaMgZnFeCu. Reaction of non metals with water. Non metals do not react with water as to react they need to displace hydrogen and for displacing they need to donate electrons to hydrogen but it is not possible as they are themselves electron acceptors.
3 Reaction with dilute. It is heavier than air. Natural sources include volcanoes hot springs and geysers and it is freed from carbonate rocks by dissolution in water and acids.
Because carbon dioxide is soluble in water it occurs naturally in groundwater rivers and lakes in ice caps and glaciers and also in seawater. CO 2 is an asphyxiant gas asphyxia. A condition arising when the body is.
Antimony is a member of group 15 of the periodic table one of the elements called pnictogens and has an electronegativity of 205. In accordance with periodic trends it is more electronegative than tin or bismuth and less electronegative than tellurium or arsenicAntimony is stable in air at room temperature but reacts with oxygen if heated to produce antimony trioxide Sb 2 O 3. Hydrogen and carbon atoms.
The chemical composition of natural gas is a function of the gas source and type of processing. It is a mixture of methane ethane propane and butane with small amounts of heavier hydrocarbons and some impurities notably nitrogen and complex sulphur compounds water carbon dioxide and hydrogen sulphide which may exist in the feed gas but are removed before. Mercaptans occur in all fractions from methane and heavier but cannot be removed by distillation because of which it requires chemical treatment for removal.
The amount generally ranges from a few ppm to several thousand ppm. Natural gas includes H 2 S CO 2 and other sulfur components such as mercaptans. The overall formula of mercaptans is RSH.
Here R means the hydrocarbon chain. LPG is heavier than air vs natural gas which is lighter than air. Both are gases at at standard temperature and pressure STP.
In examining LPG vs natural gas one of the differences between LPG and natural gas is their chemical names with LPG known as propane and natural gas known as methane. LPG vs natural gas also have different chemical formulas with Propane chemical. Form a heavier nucleus with the release of a very large amount of energy.
Energy of sun is produced by the fusion of hydrogen nuclei to form helium. It is also used to make the hydrogen bomb. Advantages of nuclear energy-i It produces a very large amount of energy per unit mass than any other.
Ii If safety measures are taken it is more environment. Burning of Sulphur or Sulphide ores in the air to produce SO 2. Conversion of SO 2 into SO 3 by reaction with air in the presence of catalyst V 2 O 5.
Absorption of SO 3 in H 2 SO 4 to give Oleum H 2 S 2 O 7. The key step is the catalytical oxidation of SO 2 with O 2 to give SO 3 in the presence of V 2 O 5. The reaction is exothermic reversible and proceeds with a decrease in volume.
Vapors are heavier than air. They will spread along the ground and collect and stay in poorly-ventilated low-lying or confined areas eg sewers basements and tanks. Hazardous concentrations may develop quickly in enclosed poorly-ventilated or low-lying areas.
Keep out of these areas. Although sulfur mustard is heavier than water small droplets float on water surfaces. Pyrophoric hazards on a chemical reaction with Hydrogen Sulfide Gas Pyrophoric Iron Sulphide forms when Hydrogen Sulfide Gas usually present in most crude reacts with rusted surfaces in the absence of Oxygen Inert conditions inside cargo tanks.
These substances can heat to incandescence in contact with air. This risk is minimized by following the correct purging procedure. It is heavier than air and will therefore sink to the ground if released from its container.
It is the toxic effect of chlorine gas that makes it a good disinfectant but it is toxic to more than just waterborne pathogens. It is also toxic to humans. It is a respiratory irritant and it can also irritate skin and mucus membranes.
Exposure to high volumes of chlorine gas fumes can cause serious. Fig 411 Filter Setup FILTER USED 1. Pre Filter and Cold Catalyst Filter Pre Filter and Cold Catalyst Filter is the first stage of filtration.
Heavier particles like pet dander and human hair are removed by this filter. The cold catalyst filter is effective at removing formaldehyde benzene ammonia hydrogen sulphide and harmful gases.