
Health Effects Since hydrogen sulfide can impair your sense of smell the first indication you may notice is burning or irritation of the eyes throat and respiratory tract. Vapors from liquefied gas are initially heavier than air and spread along ground.
Being heavier than air the gas accumulates at the bottom of poorly ventilated spaces.
Is hydrogen sulfide heavier than air. Because it is heavier than air hydrogen sulfide can collect in low-lying and enclosed spaces such as manholes sewers and underground telephone vaults. Its presence makes work in confined spaces potentially very dangerous. The health effects of hydrogen sulfide depend on how much H 2 S a worker breathes and for how long.
However many effects are seen even at low concentrations. Hydrogen sulfide is slightly denser than air. A mixture of H 2 S and air can be explosive.
Hydrogen sulfide burns in oxygen with a blue flame to form sulfur dioxide SO 2 and water. In general hydrogen sulfide acts as a reducing agent especially in the presence of base which forms SH. At high temperatures or in the presence of catalysts sulfur dioxide reacts with hydrogen sulfide to.
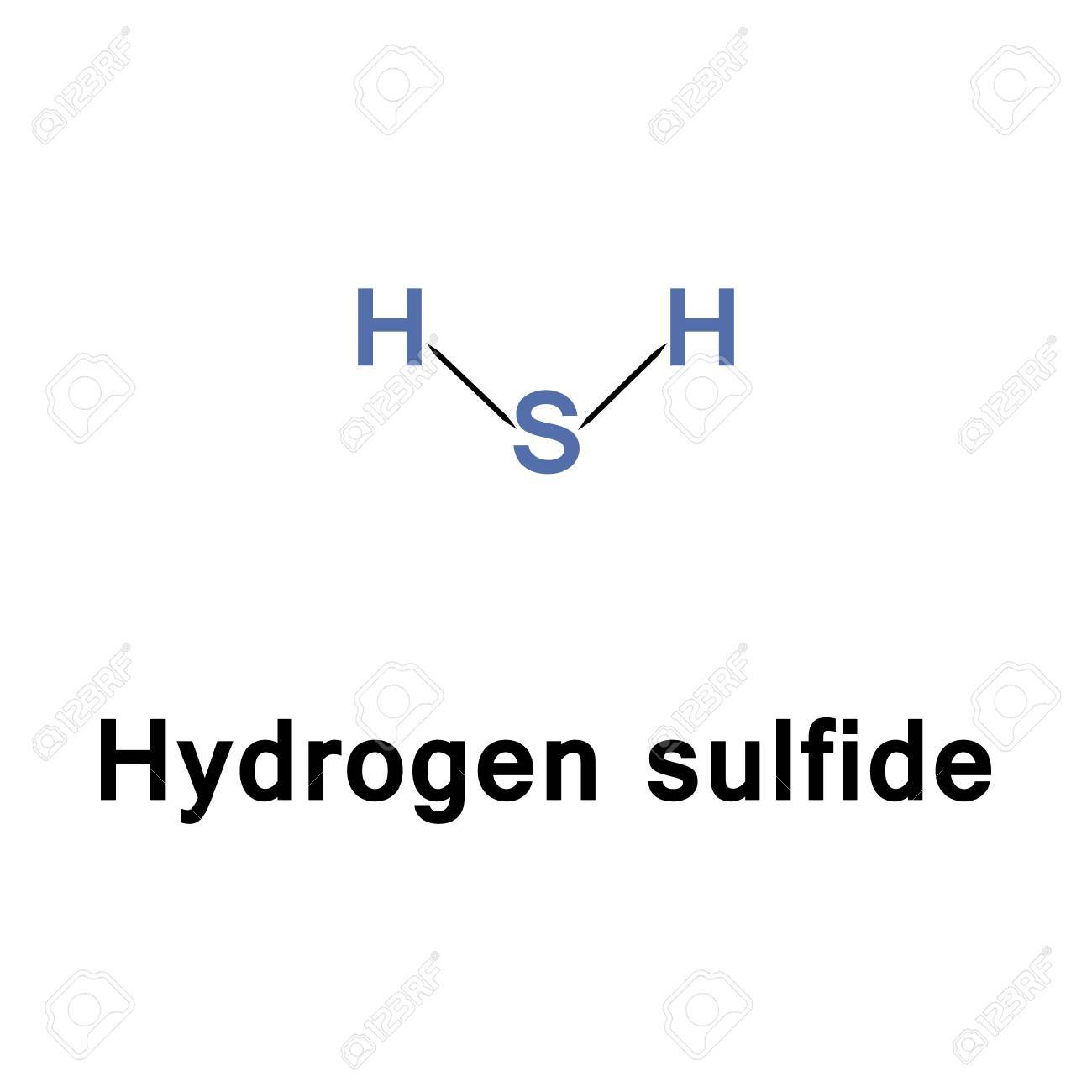
Hydrogen sulfide can also result from industrial activities such as food processing coke ovens kraft paper mills tanneries and petroleum refineriesHydrogen sulfide is a flammable colorless gas with a characteristic odor of rotten eggs. It is commonly known as hydrosulfuric acid sewer gas and stink damp. People can smell it at low levels.
Hydrogen sulfide is heavier than air and may travel along the ground. It collects in low-lying and enclosed poorly-ventilated areas such as basements manholes sewer lines under-ground telephone vaults and manure pits. For work within confined spaces use appro-priate procedures for identifying hazards monitoring and entering confined spaces.
The primary route of exposure is inhalation and. Hydrogen sulfide is slightly heavier than air and may accumulate in enclosed poorly ventilated and low-lying areas. Children exposed to the same levels of hydrogen sulfide as adults may receive larger doses because they have greater lung surface areabody weight ratios and increased minute volumesweight ratios.
In addition they may be exposed to higher levels than adults in the same. Hydrogen Sulfide or sour gas H2S is a flammable colorless gas that is toxic at extremely low concentrations. It is heavier than air and may accumulate in low-lying areas.
It smells like rotten eggs at low concentrations and causes you to quickly lose your sense of smell. Many areas where the gas is found have been identified but pockets of the gas can occur anywhere. Hydrogen sulfide is heavier than air so you should expect to find it in low areas especially sewer lines pits and cellars.
If you ignite hydrogen sulfide the fire will flash back to the source of the gas. Health Effects Since hydrogen sulfide can impair your sense of smell the first indication you may notice is burning or irritation of the eyes throat and respiratory tract. Hydrogen sulfide H 2 S is a highly toxic and flammable colorless gas with a characteristic odor of rotten eggs.
It is used in the manufacture of chemicals in metallurgy and as an analytical reagent. It is heavier than air and tends to accumulate at the bottom of poorly ventilated spaces. Although very pungent at first it quickly deadens the sense of smell.
Hydrogen sulfide occurs. H 2 S properties. SO 2 and H 2 S are very toxic gases and causes harmful injuries to humans animals and whole environment.
Inhaling H 2 S in very small amounts is enough to cause death. H 2 S toxic levels are mentioned below. Hydrogen sulfide toxicity levels.
This toxicity levels of H 2 S and effects were taken by. Hydrogen sulfide is slightly denser or heavier than air but fairly soluble in water. Hydrogen sulphide is a colourless poisonous gas that can lead to headaches even if it is inhaled in small quantities.
It has a distinct rotten egg smell. Hydrogen sulfide is a flammable gas. Chemical Properties of Hydrogen Sulphide.
A mixture of air and H 2 S can lead to an explosion. Oxygen and Hydrogen. Hydrogen gas is very rare in the Earths atmosphere 1 ppm by volume because of its light weight which enables it to escape from the atmosphere more rapidly than heavier gases.
However hydrogen is the third most abundant element on the Earths surface mostly in the form of chemical compounds such as hydrocarbons and water. Heavier than air H2S gas accumulates in low lying areas of poorly ventilated spaces. In oil and gas applications sour gas products containing H2S gas in the presence of air and moisture may form sulfuric acid capable of corroding metals.
Facility equipment including the internal surfaces of various components faces reduced durability and impact strength potentially leading to premature. If a jet of hydrogen in air impinges on platinum black the metal surface gets hot enough to ignite the gases Mellor 13251946-1947. Explosive reactions occur upon ignition of mixtures of nitrogen trifluoride with good reducing agents such as ammonia hydrogen hydrogen sulfide or methane.
Mixtures of hydrogen carbon monoxide or methane and oxygen difluoride are exploded when a spark. Will form explosive mixtures with air. Vapors from liquefied gas are initially heavier than air and spread along ground.
Hydrogen UN1049 Deuterium Hydrogen refrigerated liquid UN1966 and Methane are lighter than air and will rise. Hydrogen and Deuterium fires are difficult to detect since they burn with an invisible flame. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structureThe chemical symbol for Hydrogen is H.
With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table. Its monatomic form H is the most abundant chemical substance in the Universe constituting roughly 75 of all baryonic mass. Gases and Compressed Air - Air LNG LPG and other common gas properties pipeline capacities sizing of relief valves.
Material Properties - Material properties for gases fluids and solids - densities specific heats viscosities and more. Density - Density of different solid materials liquids and gases. Definitions and convertion calculators.
Only two stable nuclides have fewer neutrons than protons. The neutron is slightly heavier than the. Antimony is a lustrous gray metalloid it is found in nature mainly as the sulfide mineral stibnite.
Antimony compounds have been known since ancient times and were powdered for use as medicine and cosmetics often known by the Arabic name kohl. Hydrogen sulfide is heavier than air and hangs low to the ground if it is not blown away by the wind or otherwise dispersed. A top cause of air pollution occurs when manure lagoons are stirred to re-suspend solid waste releasing high concentrations of the gas into the air.
6 Hydrogen sulfide may be detected on fields where manure is sprayed for fertilizer and can be dispersed by the wind. For example among the hydrogen isotopes deuterium denoted as D or 2 H. Lighter the heavier material is the one with the higher value moreless positive vs.
Moreless negative eg -10 is more positive than -20 enriched vs. Depleted remember to state what isotope is in short supply. Eg a material is enriched in 18 O or 16 O relative to some other material.
Hydrogen sulfide is a colorless flammable gas with a strong offensive odor. It is sometimes referred to as sewer gas. Interestingly the human nose is more sensitive to H 2 S than any gas monitoring instrument we have today.
Air mixtures with as little as 0000001 H 2 S are associated with a rotten egg smell. Unfortunately however our sense of smell is not a reliable alarm - at mixing. Over-charging a vented lead acid battery can produce hydrogen sulfide H 2 S.
The gas is colorless very poisonous flammable and has the odor of rotten eggs. Being heavier than air the gas accumulates at the bottom of poorly ventilated spaces. Although noticeable at first olfactory detection between 0001-013 ppm the sense of smell deadens the sensation with time and potential victims.
In this case however amine solutions are used to remove the hydrogen sulfide. This process is known simply as the amine process or alternatively as the Girdler process and is used in 95 percent of US. The sour gas is run through a tower which contains the amine solution.
This solution has an affinity for sulfur and absorbs it much like glycol absorbing. As rain condenses the heavier isotopes of water mainly HD 16 O and H 2 18 O are preferentially removed from the air mass and into the rain and the air mass consequently becomes progressively lighter in isotopic composition ie higher concentrations of H 2 16 O. Hence the isotopic compositions of successive aliquots of rain become progressively lighter in the heavier isotope due to.
Within the troposphere are layers of clouds with methane clouds on top ammonium hydrosulfide clouds ammonia and hydrogen sulfide clouds and water clouds at the lowest pressures. Earlier snowmelt means more summer heat goes into the air and ground rather than into melting snow raising temperatures in a positive feedback effect. More dark shrubs and forest on formerly bleak tundra means still more heat is absorbed by vegetation.
Out at sea the pace is even faster. Whilst snow-covered ice reflects more than 80 of the suns heat the darker ocean absorbs up to 95 of.