
PediaSmart is a good option for children who tend to skip breakfast. What hybridization does each represent.
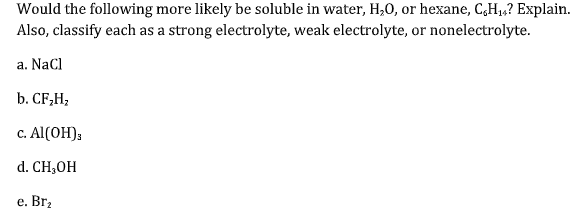
This reaction is essentially 100 complete for HCl ie it is a strong acid and consequently a strong electrolyte.
Is hexane a strong electrolyte. Request for Solution File Jul 18 2019 A strong electrolyte is defined as the electrolyte which completely dissociates into its ions when dissolved in water. The acid strength increases as the experimental pKa values decrease in the following order. HF May 27 2020 Alcohol is a base when it is combined with another strong base such as NaOH and this is the most common outcome for.
This reaction is essentially 100 complete for HCl ie it is a strong acid and consequently a strong electrolyte. Likewise weak acids and bases that only react partially generate relatively low concentrations of ions when dissolved in water and are classified as weak electrolytes. The reader may wish to review the discussion of strong and weak acids provided in the earlier chapter of.
Raoults Law is expressed by the vapor pressure equation. P solution Χ solvent P 0 solvent where P solution is the vapor pressure of the solution Χ solvent is mole fraction of the solvent P 0 solvent is the vapor pressure of the pure solvent When two or more volatile solutions are mixed each pressure component of the mixed solution is added together to find the total vapor pressure. Hexane is a nonpolar liquid with a dipole moment of zero and therefore does not significantly interact with the ions of the NaCl crystals.
A FeNO 3 3 is a strong electrolyte thus it should completely dissociate into Fe 3 and NO 3 ions. Therefore z best represents the solution. B FeNO 3 3 s Fe 3 aq 3NO 3 aq 5.
An equivalent Eq is the amount of an electrolyte that carries one mole of positive or negative charge. One mole of Na has IEq One mole of Ca2 has 2Eq. The Concepts Select the diagram that represents the solution formed by a solute that is a.
Nonelectrolyte Weak electrolyte Strong electrolyte moUc-ulJS all Practice Problems 4. KF is a. Electrolyte-gated transistors EGTs capable of transducing biological and biochemical inputs into amplified electronic signals and stably operating in aqueous environments have emerged as.
A solution containing 1 mol of a strong electrolyte will have an _____ effect on the colligative properties of the solution than 1 mol of a nonelectrolyte. A strong electrolyte will dissociate or ionize in aqueous solution and therefore the total number of dissolved solute particles is _____ for 1 mol of a strong electrolyte than for 1 mol of a nonelectrolyte solute. Water is a polar solvent and hexane C6H14 is a nonpolar solvent.
Which of the following correctly describes the solubility of the solute. A mineral oil soluble in water B CaCl2 soluble in hexane C NaHCO3 soluble in water D CCl4 soluble in water E octane soluble in water. C NaHCO3 soluble in water.
In water a substance that ionizes completely in solution is called a A weak. PediaSmart is a good option for children who tend to skip breakfast. This beverage has been scientifically formulated to function as a meal replacement providing the appropriate blend of protein fat carbohydrate vitamins minerals that will help a child start their morning nutritionally strong.
Less strong parts than wrought ones Less well known process. Ganesh Narayanan IITG Production of powders Metal powders Main constituent of a PM product. Final properties of the finished PM part depends on size shape and surface area of powder particles Single powder production method is not sufficient for all applications Powder production methods.
It is a covalent nonelectrolyte b. It is a covalent strong electrolyte c. It is a covalent weak electrolyte d.
It is soluble ionic compound e. It is an insoluble ionic compound 3. Identify each geometry shown below.
What hybridization does each represent. Or atom ion ion. The interfacial resistance decreased gradually and reached about 20 Ω for 10 days indicating the components in electrode and electrolyte react to form a passivation layer which hinders the further reactions between the two interfaces.
The dendrite penetration is also inhibited during long-term lithium depositionstripping. These results show that FBCPEs have good interfacial compatibility. The unique electronic structure and strong metal-support interaction make it a candidate support for Pt-based electrocatalyst.
Carbon is universal as support of Pt-based electrocatalyst. However it is restricted to the poor durability and bad methanol tolerance. On the contrary the chemical stability of nitrogen-doped TiO 2 contributes to the higher durability and methanol tolerance of PtN.
A 160 g sample of napthalene a non-electrolyte with a formula of C 10 H 8 is dissolved in 200 g of benzene. The freezing point of benzene is 55 C and K f 512 kgmol. What is the freezing point of the solution.
1 Determine the molality of napthalene. 160 g 1281732 gmol 00200 kg 0624155 m. 2 Determine the freezing point depression.
Δt i K f m x. Cz was further purified by column chromatography two more times using ethyl acetatehexane 595 vv and DCMhexane 12 vv as eluents respectively. After this column chromatographic.
Mar 20 2020 Soluble ionic compounds and strong acids are therefore both strong electrolytes although the latter are molecular compounds that actually react with water as indicated by the production of hydronium ion or H 3 O aq which is commonly abbreviated as H aq as shown in the second equation above. A chemical reaction is when there is a change in the molecular make up of a. We would like to show you a description here but the site wont allow us.
A FeNO33 is a strong electrolyte thus it should completely dissociate into Fe3 and The molecule POCl3 is a polar molecule because there is an uneven distribution of electrons throughout the molecule. 2019 lanashanabJHsbd1099 Sep 12 2020 Ionic and covalent bonds are the two extremes of bonding. They are insoluble in water but soluble in nonpolar solvents such as hexane gasoline.
Strong solvent-solute interactions make the process of solvation more favorable. One way to compare how favorable the dissolution of a solute is in different solvents is to consider the free energy of transfer. The free energy of transfer quantifies the free energy difference between dilute solutions of a solute in two different solvents.
This value essentially allows for comparison of. Both benzene and hexane are hydrocarbons. 1 molar NaCl solution has higher boiling point than one molar urea.
NaCl dissociates into ions in solution. A raw mango placed in concentrated salt solution loses water shrivel into pickle. The salt solution is hypotonic compared to the raw mango.
Helium is mixed with nitrogen and. Academiaedu is a platform for academics to share research papers. The carboxylate moiety of lacceroic acid 12 formed strong ionic interactions with ZN ion and accepted a hydrogen bond from the side chains of His94 Thr199 and Thr200.
Additionally the main chain amino nitrogen of Thr199 also mediated the H-bond with the carboxylate group of the compound. This suggests that the carboxylate moiety is responsible for the binding in turn for inhibitory. Academiaedu is a platform for academics to share research papers.
43 a HCOOH is a weak electrolyte. B HNO 3 is a strong electrolyte. C CH 3 CH 2 OH is a nonelectrolyte.
47 b NO 3 and c NH 4 will always be spectator ions. 49 In a redox reaction electrons are transferred from the oxidized substance to the reduced substance. In an acid-base reaction protons are transferred from.