Ethyl acetate systematically ethyl ethanoate commonly abbreviated EtOAc or EA is the organic compound with the formula CH 3 COOCH 2 CH 3. The ester we have prepared ethyl acetate ethyl ethanoate has the lowest boiling point of all the possible components in the mixture.
Calcium chloride removes water.
Is ethyl ethanoate an ester. Ethyl acetate systematically ethyl ethanoate commonly abbreviated EtOAc ETAC or EA is the organic compound with the formula CH 3 COOCH 2 CH 3 simplified to C 4 H 8 O 2This colorless liquid has a characteristic sweet smell similar to pear drops and is used in glues nail polish removers and in the decaffeination process of tea and coffee. Ethyl acetate is the ester of ethanol. Ethyl ethanoate ethyl acetate is an ester.
The general method of ester preparation can be summarised as the reversible reaction. Acid alcohol ester water. Concentrated sulphuric acid is used to catalyse the reaction and to remove water from the right hand side of the equilibrium increasing the yield of ester.
CH3COOH C2H5OH h CH3COOC2H5 H2O. Calcium chloride removes water. For my assignment I need to know ethyl ethanoate is manufactured industrially and what the raw materials are the equipment used and the scale of production but I cant find anything on the internet someone please help.
Then i i need to compare the manufacturing of ethyl ethanoate in a laboratory and industrial and what are the similarties and differences in the equipment and techniques used. The ester we have prepared ethyl acetate ethyl ethanoate has the lowest boiling point of all the possible components in the mixture. Therefore ethyl acetate will be the first fraction collected as the distillate.
A sharp boiling point is an indication of the purity of the ester. An ester is a chemical compound derived from an acid organic or inorganic in which at least one OH hydroxyl group is replaced by an O alkyl group as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides are fatty acid esters of glycerol.
They are important in biology being one of the main classes of lipids and comprising the bulk of animal fats and vegetable. A common ester - ethyl ethanoate. The most commonly discussed ester is ethyl ethanoate.
In this case the hydrogen in the -COOH group has been replaced by an ethyl group. The formula for ethyl ethanoate is. Notice that the ester is named the opposite way around from the way the formula is written.
The ethanoate bit comes from ethanoic acid. The ethyl bit comes from the ethyl group on the. An ester is a chemical compound that is formed when an organic acid reacts with an alcohol.
Esters frequently have distinctive odors and are naturally occurring flavor and fragrance chemicals in many fruits and plants. The ester ethyl acetate ethyl ethanoate is prepared and purified by distillation. The preparation of ethyl ethanoate Method.
A mixture of ethanoic acid ethanol and concentrated sulfuric acid is gently heated by either a water bath or an electric. Ethyl acetate ethyl ethanoate is an ester. The hydrogen on the carboxyl group of acetic acid is replaced with an ethyl group.
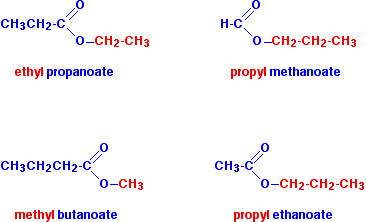
Other examples of esters include ethyl propanoate propyl methanoate propyl ethanoate and methyl butanoate. Glycerides are fatty acid esters of glycerol. Fats and oils are examples of esters.
The difference between them is the melting point of their. A common ester - ethyl ethanoate. The most commonly discussed ester is ethyl ethanoate.
In this case the hydrogen in the -COOH group has been replaced by an ethyl group. The formula for ethyl ethanoate is. Notice that the ester is named the opposite way around from the way the formula is written.
The ethanoate bit comes from ethanoic acid. The ethyl bit comes from the ethyl group on the. To give you an example of an ester we can talk about ethyl ethanoate.
Here the hydrogen in the -COOH group is replaced by an ethyl group. You can find the ethyl ethanoate formula below. In this case the ester is usually named in the opposite way around from the way the formula is written.
In this case ethanoate part comes from ethanoic acid and the ethyl part comes from the. SOLUTION 10 IN CCl4 FOR 3800-1300 10 IN CS2 FOR 1300-650 10 IN CCl4 FOR 650-250 CM-1 VERSUS SOLVENT. PERKIN-ELMER 521 GRATING Instrument parameters.
GRATING CHANGES AT 2000 630 CM-1. 0012 0012 AND 0010 CM CsBr CELL Resolution. A common ester - ethyl ethanoate.
The most commonly discussed ester is ethyl ethanoate. In this case the hydrogen in the -COOH group has been replaced by an ethyl group. The formula for ethyl ethanoate is.
Notice that the ester is named the opposite way around from the way the formula is written. The ethanoate bit comes from ethanoic acid. The ethyl bit comes from the ethyl group on the.
Ester second name -oate. Methyl ethanoate See bottom panel on left Example on bottom left - Esters. In compounds where oxygen breaks a continuous chain of carbons the compound must be named in two parts.
In esters divide the compound between the carbon double bond oxygen and the part with the single bond oxygen. The part with the single bond oxygen is named first with the root plus -yl. To make a small ester like ethyl ethanoate you can gently heat a mixture of ethanoic acid and ethanol in the presence of concentrated sulphuric acid and distil off the ester as soon as it is formed.
This prevents the reverse reaction happening. It works well because the ester has the lowest boiling point of anything present. The ester is the only thing in the mixture which doesnt form.
Ester names are derived from the parent alcohol and acid. For example the ester formed by ethanol and ethanoic acid is known as ethyl ethanoate. Ethanol is reduced to ethyl while ethanoic acid is reduced to ethanoate Other examples of ester names include methyl propanoate from methanol and propanoic acid and butyl octanoate from butane and octanoic acid.
The preparation of an organic liquid compound eg. The preparation of an ester. Is also expected that candidates will be aware of the procedures involved in the purification and re-distillation at an appropriate temperature of the product obtained.
The Preparation of Ethyl Ethanoate. Mix 50cm3 of ethanol and 50cm3 of glacial ethanoic acid thoroughly in a 250cm3 round-bottomed. Ethyl Acetate is a most familiar ester of ethanol which you can easily remember by its regular use in our daily life.
It is also called as ethyl ethanoate commonly abbreviated EtOAc or EA. It is one of the ingredients responsible for the characteristic smell of a banana flower or overripe fruit has high levels of ethyl acetate. It is manufactured on a large scale for use as a solvent.
The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. The primary aim of the cards is to promote the safe use of chemicals in the workplace. The main target users are workers and those responsible for occupational safety and health.
The ICSC project is a common undertaking between the World Health Organization WHO and. Ethanol reacts with acetic acid to give ethyl ethanoate which is an ester compound. Concentrated sulfuric acid hydrolysis the formed ethyl ethanoate to ethanoic acid and ethanol.
Ethanol to ethanoic acid reaction. Ethanol reacts with. Ethyl acetate systematically ethyl ethanoate commonly abbreviated EtOAc or EA is the organic compound with the formula CH 3 COOCH 2 CH 3.
This colorless liquid has a characteristic sweet smell similar to pear drops and is used in glues nail polish removers decaffeinating tea and coffee and cigarettes see list of additives in cigarettes. Ethyl acetate is the ester of ethanol and. B ester c ketone d carboxylic acid e alcohol 26.
The compound given below is called _____. A butyl acetate b ethyl pentanoate c propyl pentanoate d ethyl butanoate e butyl ethanoate 27. The compound illustrated below is called _____.
A acetamide b formyl acetamide c dimethyl acetate d NN-dimethylformamide e. Esters are formed by the condensation reaction between an alcohol and a carboxylic acid. This is known as esterification.
In a condensation reaction two molecules join and produce a. Ethenyl ethanoate and an acrylic ester for example methyl 2-methylpropenoate are then co-polymerized to form a random array in which these groups link into a linear chain. Other acrylic esters used as co-monomers with ethenyl ethanoate are ethyl propenoate butyl propenoates or a co-polymer of butyl propenoate and methyl 2-methylpropenoate.
Ethanoic acid Ethanol Ethyl Ethanoate Uses of Esters Esters can have pleasant smells Esters are sweet smelling compounds that can be used in perfumes and flavourings. For use in perfumes they need to be non toxic soluble in solvent such as ethanol volatile turns into gas easily and not react with water. Esters can be used as solvents for polar organic substances.
Ethyl ethanoate is used.