
To denote this distinct chemical property a mixture of water with an acid is given a name derived from. When dissolved in water.
A 697 g of sodium acetate dissolved in 1 L of water B 148 g of sodium acetate dissolved in 300 mL of water C 450 g of sodium acetate dissolved in 500 mL of water D 98 g of sodium acetate dissolved in 200 mL of water.
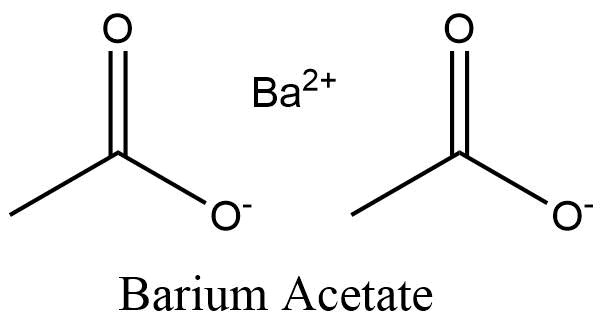
Is barium acetate acidic when dissolved in water. Water bath Sodium Chloride Ethyl Acetate 2 O 3 Chloroform Ethanol Methanol Sulphuric Acid Ethanolic Sodium Hydroxide-01 mollit Sodium Hydroxide Ethanol Methylene Blue Neutral Solution Methylene Blue Acidic Solution Buffer Solution pH 10 Sodium Hydrogen Carbonate Anhydrous Sodium Carbonate. The hydroxides of calcium strontium and barium are moderately soluble. Ammonium hydroxide does not exist.
Ammonium hydroxide is a misnomer for aqueous ammonia NH 3 aq. Reaction with nitric acid. Add nitric acid to the compound and observe any reaction that occurs.
If the compound dissolved in water it should dissolve in nitric acid. Solubility of barium iodate in presence of barium nitrate. Of the buffer solution.
For example if both sodium acetate and acetic acid are dissolved in the same solution they both dissociate and ionize to produce acetate ions. Sodium acetate is a strong electrolyte so it dissociates completely in solution. Acetic acid is a weak acid so it only ionizes slightly.
According to Le Chateliers. 040 gram of sodium hydroxide is dissolved in enough water to make 500 ml of solution at 25circC. Given that the atomic mass is 1008 for hydrogen 15999 for oxygen and 2299 for sodium calc.
The chemistry of these compounds is explored in more detail in later but for now it will suffice to note that many acids release hydrogen ions H when dissolved in water. To denote this distinct chemical property a mixture of water with an acid is given a name derived from. With zinc uranyl acetate.
Precipitation ignition volatilization with HF SO4. Total Dissolved and Suspended Solids. Definitions Total solids TS is generally defined as all matter in a water or wastewater sample that is not water.
Because solids are not a specific chemical compound but rather a diverse collection of dissolved and. When dissolved in water. However enables weaker bases such as carboxylates.
For example sodium acetate is a weak base. A strong base is a basic chemical compound that can remove a proton H from or deprotonate a molecule of even a very weak acid such as water in an acidbase reaction. Common examples of strong bases include hydroxides of alkali metals and alkaline.
The extent to which a substance may be dissolved in water or any solvent. The weak acid hydrogen hypochlorite reacts with water b a solution of barium hydroxide is neutralized with a solution of nitric acid. Solution a The two reactants are provided HOCl and H 2 O.
Since the substance is reported to be an acid its reaction with water will involve the transfer of H from HOCl to H 2. If the solubility of sodium acetate is 76 grams per 100 grams of water which of the following solutions would be considered supersaturated. A 697 g of sodium acetate dissolved in 1 L of water B 148 g of sodium acetate dissolved in 300 mL of water C 450 g of sodium acetate dissolved in 500 mL of water D 98 g of sodium acetate dissolved in 200 mL of water.
1476 g of N_2 acetamido-2-aminoethane-sulfonic acid potassium salt is dissolved in 7177 mL of water. 1254 mL of HCI is added to the solution resulting in a pH of 735. Add aqueous barium chloride BaCl 2 to the water extract.
Formation of a white precipitate which disappears when diluting hydrochloric acid HCl is added. Sulphide S 2- i Add sodium nitroprusside to the water extract ii Add aqueous lead acetate to the water extract i The solution turns purple or violet ii Formation of a black precipitate. Nitrite NO 2 Boil a mixture of the.
Acidic drainage from mines or acid rain may cause an increase in the dissolved Al content of the surrounding water bodies Cronan and Schofield 1979. Filipek et al 1987. The use of Al compounds as coagulating agents in the treatment of water for drinking could increase its Al content Qureshi and Malmberg 1985.
Cech and Montera 2000. A mixture of BaCl2 and NaCl is analyzed by precipitating all the barium as BaSO4. After addition of an excess of Na2SO4 to a 3725-g sample of the mixture the mass of precipitate collected is 2734 g.
What is the mass percentage of barium chloride in the mixture. If water from the hot water bath spills into the beaker there will be a drasticdecrease in the yield of alum. Place the 100-mL beaker containing the aluminum into the hot water in the Styrofoam cup and transfer everything to the hood.
Slowly and carefully add 25 mL of the 14 M KOH solution to the aluminum. No open flames can be present in lab while the reaction between KOH and Al is. Barium MCL 2 mgL EPA US 2006 occurs naturally in some aquifers that serve as sources of ground water.
It generally gets into drinking water after dissolving from naturally occurring minerals in the ground. It may damage heart and cardiovascular system and is associated with high blood pressure in laboratory animals such as rats exposed to high levels during their lifetimes Brenniman et al. To each cylinder add 2 ml of acetate buffer pH 35 mix.
Take 10 ml sample add 01 ml of 2 M Hydrochloric acid and 01 ml of barium chloride solution. The appearance of the solution does not change for at least 1 hour. Take 100 ml sample add 10 ml of 1 M sulphuric acid and 01 ml of 002 M potassium permanganate and boil for 5 minutes the solution should.
-acetate OAc HSO 3. Especially when dissolved in water which is most frequently the case The word hydrogen is omitted and the word acid is used at the end. The suffix is determined from the name of the anion portion.
Compound name Acid name Example Compound Name Acid name -ate -ic acid HClO 3 hydrogen chlorate chloric acid H 2SO 4 hydrogen sulfate sulfuric acid -ite. BSodium hydroxide solution is treated with acetic acid to form sodium acetate and water. CEthanol is warmed with ethanoic acid to form ethyl acetate in presence of cone.
H _ 2 SO_ 4. DEthene is burnt in presence of oxygen to form carbon dioxide water and releases heat and light. Usually homogeneous catalysts with substrates are dissolved in a solvent.
The effect of H on the esterification of carboxylic acids such as the formation of methyl acetate from acetic acid and methanol is one example of homogeneous catalysis. Hydroformylation hydrosilylation hydrocyanation involve high-volume processes requiring a homogeneous catalyst. Homogeneous catalysis is frequently.
Acidic dissolution of crushed shells was carried out by processing them in ultrapure water Merck H 2 O 2 bidistilled ultrapure water Aldrich HNO 3 HNO 3 HCl and finally in HNO 3 at 80 C in Savillex Teflon containers housed in separate evaporation chambers class A 100 located inside the cleanroom class ISO A 10000. This made it possible to dissolve only the carbonate and organic. Method 4500-Sulfate ion F.
Automated Methylthymol Blue Method for the determination of sulfate ions in water and wastewater. Barium sulfate is formed by the reaction of the sulfate ion with barium chloride at a low pH. At high pH excess barium reacts with methylthymol blue to produce a blue chelate.
The uncomplexed methylthymol blue is gray. The amount of gray uncomplexed methylthymol blue. Reaction of sodium hydrogen carbonate with acetic acid forms sodium acetate and water with carbon dioxide CO2 gas.
Common salt besides being used in kitchen can also be used as the raw material for making i washing soda ii bleaching powder. I and ii b. I ii and iv c.
I ii and iii d. I iii and iv Answer. It is not used as a solvent but may be used in minor quantities in order to dissolve the colour.
It is mostly used to aid in mould release and prevent the rub-out of the. The extent to which a substance may be dissolved in water or any solvent. The weak acid hydrogen hypochlorite reacts with water b a solution of barium hydroxide is neutralized with a solution of nitric acid.
Solution a The two reactants are provided HOCl and H 2 O. Since the substance is reported to be an acid its reaction with water will involve the transfer of H from HOCl to H 2. We would like to show you a description here but the site wont allow us.