Ferrous Sulfate 7 Fe2 SECTION 1. Please see warning notes below.

Please see warning notes below.
Ironcopper sulfate. Zinc sulfuric acid zinc sulfate hydrogen Zn H 2SO 4 ZnSO 4 H 2 13. Calcium oxide water calcium hydroxide CaO H 2O CaOH 2 14. Iron copper I nitrate iron II nitrate copper Fe 2CuNO 3 FeNO 3 2 2Cu 15.
Potassium oxide water potassium hydroxide K 2O H 2O 2KOH. Copper sulfate is an algaecide bactericide and fungicide. When it is mixed with calcium hydroxide it is known as Bordeaux mixture.
1 The International Union of Pure and Applied Chemistry IUPAC name for this active ingredient is copper 2 sulfate or copper II sulfate. Other names include copper 2 tretraoxidosulfate or copper II tretraoxidosulfate. Formulations include basic.
Two observe two different single displacement reactions. When zinc is added to copper II sulfate a single displacement reaction will take place creating a solid copper and zinc sulfate. When zinc is added to hydrochloric acid hydrogen gas will be released a solid zinc chloride will be formed.
Hydrochloric acidZincCopper II sulfateTest. Ferrous Sulfate 7 Fe2 SECTION 1. CHEMICAL PRODUCT AND COMPANY INFORMATION Product Name.
Ferrous Sulfate 7. To avoid product degradation and equipment corrosion do not use iron copper or aluminum containers or equipment. Avoid contact with strong acids or bases and excessive heat.
An excess of copper II sulfate solution to make sure that all the iron is reacted will be added to a known amount of iron. The metallic copper produced will be weighed. These weighings will be used to calculate the moles of iron used and the moles of copper formed.
If equation 1 is correct the moles of copper should equal the moles of iron. If equation 2 is correct we should obtain 1. The copper sulfate test is a visual method of determining the existence of free iron in specifically stainless steel.
In many cases the copper sulfate test is used as a passfail examination of a passivation process on a part or stock material but it can also be used as a test to determine if passivation is necessary in the first place. The order of reactivity is. Magnesium iron copper.
This is because magnesium displaced copper and iron iron displaced copper only but copper could not displace magnesium or iron. Magnesium sulfate Zinc sulfate. Magnesium displaces three metals zinc displaces two metals iron displaces one metal.
The chemical compounds used in the experiments including magnesium chloride hexahydrate MgCl 2 6H 2 O AR magnesium hydroxide MgOH 2 AR magnesium oxide MgO AR ammonium chloride NH 4 Cl AR copper sulfate CuSO 4 AR and ferric chloride FeCl 3 AR were purchased from Aladdin Company Shanghai China. Sodium hydroxide NaOH and hydrochloric acid HCl solutions at the. The nail is coated with a layer of copper while the blue copper sulfate solution has turned greenish.
The green solution is a solution of iron sulfate. Fe CuSO 4 Cu FeSO 4 Most metals corrode when they are exposed to atmosphere. For example the iron gets rusty after sometime if it is not painted.
In this video well balance the equation Fe CuSO4 Cu FeSO4 and provide the correct coefficients for each compound. To balance Fe CuSO4 Cu FeSO. Sulfate radical has a higher half-life than hydroxyl radical.
Besides cobalt and iron copper and manganese oxides in nano-scale have been considered in activation of PMS. Manganese oxides in nano size MnO Mn 3 O 4 MnO 2 Mn 2 O 3 and their composites with other materials are attractive for researchers in terms of stability low cost high efficiency multi-valence nature and being. BOD Biochemical Oxygen Demand Arsenic.
Vitamin C L-Ascorbic Acid Hydrazine. Product List Anyone can use it anywhere. We make the water test easier and simpler.
We continue to support aquatic conservation through providing the simplified water. Iron copper and calcium supplements may compete with zinc for absorption so only take these if prescribed by a doctor or take them at a separate time from when you consume foods rich in zinc. Zinc from supplements is more easily absorbed than zinc from foods when you first start consuming the supplements.
However absorption decreases as your body gets used to the. Chloride Sulfate Nitrite Nitrate Phosphate Glycolate Formate Acetate Oxalate Organic Acid and Azoles by HPLCBenzoate 2-Ethylhexanoic Acid Sebacic Acid Octanoic Acid p-Toluic Acid Mercaptobenzothiazole Tolytriazole Benzothiazole NAP-Free or NAPS-Free P-OAT North American European Formulated Coolants Typical color. Red Heavy- and light-duty diesel natural gas.
Tannins Iron Copper Manganese Natural deposits Treatment Filtration Distillation Reverse osmosis Ozonisation. Odour IS 10500-1991 Unobjectionable Risks or effects Rotten egg Musty Chemical Sources Chlorine Hydrogen sulfide Organic matter Septic contamination Methane gas Treatment Activated carbon Air stripping oxidation Filtration. PH IS 10500-1991 Desirable 65 85.
Very limited evidence of effectiveness was found for the treatment of primary dysmenorrhea with zinc sulfate 50 mg daily 3 months compared to placebo or no treatment 1 randomized clinical trial n 99 and no difference in efficacy was identified between zinc sulfate 220 mg and ginger powder 250 mg 3 times a day for 4 days starting the day before menstruation to day 3 of. Standards related to odor and taste. Chloride Copper Foaming Agents Iron Manganese pH Sulfate Threshold Odor Number TON Total Dissolved Solids Zinc.
Color may be indicative of dissolved organic material inadequate treatment high disinfectant demand and the potential for the production of excess amounts of disinfectant by-products. Inorganic contaminants such as metals are. B copperII sulfate C ironII chloride D ironII sulfate 22 Which statement about the Periodic Table is correct.
A Most metallic elements are on the left. B Elements in the same period have the same number of outer electrons. C Elements on the left are usually gases.
D The relative atomic mass of the elements increases from right to left. 23 The diagram shows elements W X Y and Z in a. Iron copper nitrate — iron nitrate and copper metal.
Bromine potassium iodide — potassium bromine and iodine. Metals combined with acids are almost always single replacement chemical reactions. When they react the metal takes one element from the acid leaving a single element behind.
Reactive metals such as lithium potassium and sodium all have strong reactions to water while. However it is more effective to simply buy copper sulfate. Again this is.
The measurements are the same as for iron. Please see warning notes below. As a mordant tin has a tendency to be very harsh on the wool or yarn and can make it quite brittle.
Small amounts are recommended. However when used correctly tin leaves a clear and very fast color. Because of the.
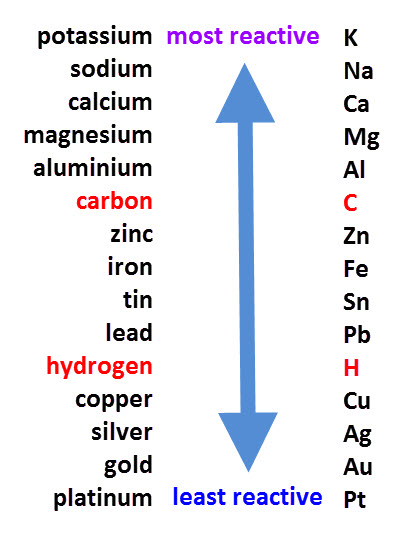
217 know the order of reactivity of these metals. Potassium sodium lithium calcium magnesium aluminium zinc iron copper silver gold. 218 know the conditions under which iron rusts.
219 understand how the rusting of iron may be prevented by. Barrier methods galvanising and sacrificial protection. 220 in terms of gain or loss of oxygen and loss or gain of electrons understand.
For iron copper and tin mordants use 12 ounce two teaspoons per pound of fiber. When ready to dye completely wet the fabric or yarn with warm water. Squeeze gently to extract excess water.
Add the fabric or yarn to the water and mordant solution stirring gently. Be sure the fabric is open and every surface is exposed to the water. Heat the pot to 180 to 200 F and keep it at that.
Aluminum Sulfate 10 B B1 A B B B B B A D A 1A A B B A A A1 A A A A A A A A A A A A A Alums NA A A1 C B A D. Buna N Nitrile Cast Iron Copper CPVC EPDM Hastelloy -C PVDF Kynar Hytrel TPE Kel-F PCTFE HDPE LDPE Natural Rubber Neoprene CR Noryl PPO Nylon PA Polycarbonate PC Polypropylene PP PTFE PVC Silicone VMQ Titanium Tygon Viton FKM Amines A A NA D D B B D. Sulfide British English also sulphide is an inorganic anion of sulfur with the chemical formula S 2 or a compound containing one or more S 2 ions.
Solutions of sulfide salts are corrosive. Sulfide also refers to chemical compounds large families of inorganic and organic compounds eg. Lead sulfide and dimethyl sulfide.
Hydrogen sulfide H 2 S and bisulfide SH are the conjugate.