For example intramolecular hydrogen bonding occurs in ethylene glycol C 2 H 4 OH 2 between its two hydroxyl groups due to the molecular geometry. Answer a An ideal solution all intermolecular interactions are the same.
Ethylene glycol and Phthalic acid.
Intermolecular forces ethylene glycol. Ethylene glycol IUPAC name. Ethane-12-diol is an organic compound with the formula CH 2 OH 2. It is mainly used for two purposes as a raw material in the manufacture of polyester fibers and for antifreeze formulations.
It is an odorless colorless sweet-tasting toxic viscous liquid. Ethylene glycol is produced from ethylene ethene via the intermediate. Intermolecular forces also cause a phenomenon called capillary action.
Will the ethylene glycol be pulled up into the tube by capillary action or pushed down below the surface of the liquid in the beaker. What will be the shape of the meniscus convex or concave. Capillary action will pull the ethylene glycol up into the capillary.
The meniscus will be concave. Intermolecular attractive forces collectively referred to as van der Waals forces are responsible for the behavior of liquids and solids and are electrostatic in nature. Dipole-dipole attractions result from the electrostatic attraction of the partial negative end of one dipolar molecule for the partial positive end of another.
The temporary dipole that results from the motion of the. The molecular mass of butanol C 4 H 9 OH is 7414. That of ethylene glycol CH 2 OHCH 2 OH is 6208 yet their boiling points are 1172 C and 174 C respectively.
Explain the reason for the difference. On the basis of intermolecular attractions explain the differences in the boiling points of nbutane 1 C and chloroethane 12 C which have similar molar masses. Intermolecular forces are electrostatic in nature.
That is they arise from the interaction between positively and negatively charged species. Like covalent and ionic bonds intermolecular interactions are the sum of both attractive and repulsive components. Because electrostatic interactions fall off rapidly with increasing distance between molecules intermolecular interactions are most.
HOCH 2 CH 2 OH. CH 3 CO 2 H. H 2 NCH 2 CH 2 NH 2.
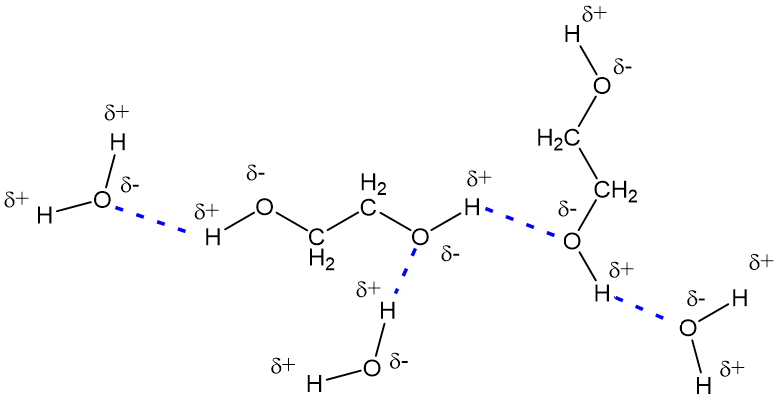
Alcohols boil considerably higher than comparably sized ethers first two entries and isomeric 1º 2º 3º-amines respectively show decreasing boiling points with the two hydrogen bonding isomers being substantially higher boiling. The ethylene glycol can form hydrogen bonds on both ends of the molecule resulting in much stronger intermolecular forces and a higher boiling point. Which of the following materials is likely to have a no dipole-dipole forces but the largest London dispersion forces b the largest dipole-dipole intermolecular forces.
I 2 He H 2 S H 2 Te. For example intramolecular hydrogen bonding occurs in ethylene glycol C 2 H 4 OH 2 between its two hydroxyl groups due to the molecular geometry. Intermolecular hydrogen bonds occur between separate molecules in a substance.
They can occur between any number of like or unlike molecules as long as hydrogen donors and acceptors are present. The low molecular size of a plasticizer allows it to occupy intermolecular spaces between polymer chains reducing secondary forces among them. In the same way these molecules change the three-dimensional molecular organization of polymers reducing the energy required for molecular motion and the formation of hydrogen bonding between the chains.
As a consequence an increase in the free. The relative strength of intermolecular forces such as ionic hydrogen bonding dipole-dipole interaction and Vander Waals dispersion force affects the boiling point of a compound. The influence of these forces depends on the functional group present.
We can explain the effect of these forces on the boiling point of compounds with the help of some examples. Consider butane and its three. The main members include oligomeric alkyl PEO surfactants amphiphilic block copolymers eg PEO-PPO-PEO sorbitan esters etc.
Commercially available triblock copolymers from BASF Company are listed in Figure 5The grid profiles of respective triblock copolymers with various molecular weight ranges of the hydrophobic parts are plotted as a function of the percentage of hydrophilic sections. They have strong intermolecular forces and thus have crystalline nature. Some examples include polyamides polyesters etc.
Ethylene glycol and Phthalic acid. Manufacture of paints and lacquers. For making combs electrical switches.
How to prepare for Amines. This chapter is part of organic chemistry. It is completely theory-based.
You are not supposed. Classify each substance based on the intermolecular forces present in that substance. Hydrogen bonding dipole-dipole and dispersion-HF Dipole-dipole and dispersion only-HCl-CO Dispersion only-CO2.
In the context of small molecules with similar molar masses arrange the intermolecular forces by strength. Strongest-hydrogen bonding-dipole-dipole interactions-London dispersion forces Weakest. Ethylene is a stable molecule that polymerizes only upon contact with catalysts.
The conversion is highly. The mostly linear molecules pack together well so intermolecular forces are stronger than in highly branched polymers. HDPE can be produced by chromiumsilica catalysts ZieglerNatta catalysts or metallocene catalysts.
By choosing catalysts and reaction conditions the small amount. Ethylene glycol 1630 349 Glycerol 2110 362 Water. The intermolecular forces in linseed oil are primarily due to dispersion forces with practically no hydrogen bonding involved.
These polar configurations are perfectly matched by the intermolecular forces between chloroform molecules thus encouraging interpenetration and swelling of the linseed oil polymer. Acetone and methyl ethyl. The intermolecular forces between solute and solvent particles must be strong enough to compete with those between solute particles and those between solvent particles.
Solutions How Does a Solution Form. As a solution forms the solvent pulls solute particles apart and surrounds or solvates them. Solutions How Does a Solution Form If an ionic salt is soluble in water it is because the ion.
Describe the intermolecular forces for each of the solutions a b and c that would cause the behavior shown in each case. Answer a An ideal solution all intermolecular interactions are the same. B Sovent-solvent and solute-solute intermolecular forces are greater than the solvent-solute intermolecular forces.
It makes it harder to. Since the surface tension is a manifestation of intermolecular forces it may be expected to be related to other properties derived from intermolecular forces such as work of cohesion internal pressure and compressibility. 1 Relationship between work of cohesion and surface tension Grunberg 1949 molecular vol.
A V N 23 A moleculesunit surf. N V V molar. Alcohol - alcohol - Physical properties of alcohols.
Most of the common alcohols are colourless liquids at room temperature. Methyl alcohol ethyl alcohol and isopropyl alcohol are free-flowing liquids with fruity odours. The higher alcoholsthose containing 4 to 10 carbon atomsare somewhat viscous or oily and they have heavier fruity odours.
Boiling points of alkanes are higher than expected because of the presence of intermolecular hydrogen bonding in the polar molecules. The boiling point decreases in the order 1 2 3 as the van der Waals forces of attraction decreases Physical Properties of Phenols. These are colourless liquids or crystalline solids but.
What intermolecular forces are shared between CH₂Cl₂ and CHCl₃. Ethylene glycol C₂H₆O₂ is used as an additive to the water in your automobile to lower its freezing point. A solution of ethylene glycol in water has a freezing point of -580C.
What is the mole fraction of ethylene glycol in this solution. Kf for water is 186Ckgmol. You dissolve a 300 g.
These two forces balance out each other as described in Equation 1 for the physical situation in terms of the Gibbs free energy. G total G elastic G mixing 1 where ΔG elastic comes from the elastic stored forces in the extended polymer chains contained in the gel networks. ΔG mixing is the result from the mixing between fluid molecules with the polymer chains.
Classification Based on Molecular Forces. These are rubber-like solids weak interaction forces are present. Strong tough high tensile strength and strong forces of interaction are present.
For example nylon -6 6. These have intermediate forces of attraction. For example polyvinyl chloride.
Thus as temperature increases the average intermolecular forces decrease. The actual manner in which the two quantities vary is nonlinear and changes abruptly when the liquid changes phase. Viscosity is normally independent of pressure but liquids under extreme pressure often experience an increase in viscosity.
Since liquids are normally incompressible an increase in pressure doesnt. The intermolecular forces between polysaccharide chains of chitosan are hydrogen hydrophobic and ionic interactions. These interactions are influenced by molecular weight and ionic strength.
Cross-linking of chitosan polymers is necessary to improve chitosan properties such as stability and durability for the aim of drug delivery. Chitosan based hydrogel networks are categorized based on the.