It plays a major role in biological molecules. For manuscripts that report new compounds or materials data must.
Until 1978 lead compounds were an important component of many paints.
Inorganic compounds are obtained from. Some inorganic compounds e. G rocks minerals cant be obtained as single crystals. In these cases X-ray powder diffraction can be used to obtain the dimensions of the unit cell for use in identification there is a large indexed catalog of lattice constants for many minerals.
Inorganic compounds are found in nature as minerals. Soil may contain iron sulfide as pyrite or calcium sulfate as gypsum. Although some inorganic species can be obtained in pure form from nature most are synthesized in chemical plants and in the laboratory.
Inorganic synthetic methods can be classified roughly according to the volatility or solubility of the component reactants. Whereas inorganic compounds are obtained from the natural processes which are not related to any of the life forms on earth or any result of human experiments which are conducted in laboratories. The difference between organic and inorganic compounds does not end with the presence or the absence of carbon atoms in them.
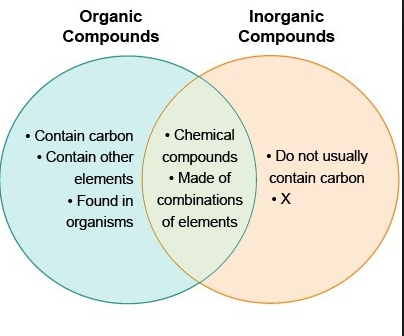
These have characteristics of both the types of compounds which are said. Difference Between Organic And Inorganic Compounds - Organic compound is a chemical compound of living things which contains Carbon and carbon atoms because of their relations with organisms. Inorganic compound is a chemical compound which is the opposite of an organic compound.
It can be considered as a compound which does not contain carbon to hydrogen bond which calls C-H bond. The inorganic compounds on the other hand do not usually contain carbon atoms nor hydrogen-carbon bonds typical of. A powerful explosive is obtained by mixing concentrated nitric acid sulfuric acid and glycerin.
Lactic acid C 3 H 6 O 3. Indispensable in energizing processes of the human body at low oxygen concentrations the production of glucose via lactic fermentation. Vitalism taught that these organic compounds were fundamentally different from the inorganic compounds that could be obtained from the elements by chemical manipulations.
Vitalism survived for a while even after the formulation of modern ideas about the atomic theory and chemical elements. It first came under question in 1824 when Friedrich Wöhler synthesized oxalic acid a compound. Inorganic and organometallic compounds.
A new chemical substance molecule or extended solid should have a homogeneous composition and structure. New chemical syntheses must unequivocally establish the purity and identity of these materials. Where the compound is molecular minimum standards have been established.
For manuscripts that report new compounds or materials data must. The results obtained indicated that this method is applicable to the entire range of test strains bacterial and fungal. Considering the inorganic compounds preservative mechanism in particular sulfites derivatives bacteria are the most sensitive.
Additionally sulfites are active against acetic acid bacteria lactic acid bacteria and Gram-negative enteric pathogens. SO 2 H 2 O. The substances obtained as a result of the reaction between an acid and a base are called Salts.
The table salt of sodium hydroxide is one of the typical examples of salts. Oxides The compounds which consist of one oxygen atom called Oxides. Types of Reactions and Examples of Inorganic compounds.
There are about four types of chemical reactions of Inorganic chemistry namely combination. Inorganic compounds are ionic compounds. Some carbon compounds are not considered to be organic mostly for historical reasons such as CO CO2 diamond graphite and salts of carbon-containing polyatomic ions eg CO 3 2- CN-.
Inorganic chemistry is the study of the other elements and non-carbon containing compounds. Hetero atoms N F Cl Br I and S are sorted in the same way as for inorganic compounds. The column labeled substance gives the systematic name the chemical formula trivial names if.
Very few of the concepts that enabled chemists to understand and manipulate the chemistry of inorganic compounds were applicable to organic compounds. This great difference in chemical behaviour between the two classes of compounds was thought to be intimately related to their origin. Inorganic substances could be extracted from the rocks sediments or waters of the Earth whereas organic.
Inorganic Chemistry Atkins ShriverPDF. Initially H 2 O molecule is in configuration I lying flat on the plane of the paper and after rotating it through an angle θ 180 0 about an axis passing through O atom Z-axis and having HOH angle the configuration II will be obtained. The configuration II is equivalent to configuration I but not identical.
By another similar rotation about Z-axis on configuration II the molecule. A higher percentage of successful grafts can be obtained in polymer-grafted inorganic particles by initiating the graft polymerization from initiating groups placed on the particles surfaces. The polymerization processes which may include radical anionic and cationic polymerization methods involves propagation of the grafted polymers from the surface of the particle.
PSi chelate hydrido iron dinitrogen complex 3 obtained from reaction of preligand L3 with FePMe 3 4 reacted with dihydrogen to deliver iron dihydride 7. Complex 3 is an efficient catalyst for silylation of dinitrogen with KC 8 as a reductant. The zeolite formation mechanism was studied systematically and the intrinsic role of inorganic cation on the synthesis was proposed.
The hydrophobicity and VOCs adsorption performance of the obtained material were investigated and compared with the ones synthesized by fluoride route and by post-synthesis dealumination. Organic forms are a very diverse group of nitrogen-containing organic molecules including simple amino acids through to large complex proteins and nucleic acids in living organisms and humic compounds in soil and water. Being a mechanical engineer my knowledge of organic chemistry is very limited our curriculum included only 1 semester of inorganic chemistry.
I dont know how to name these compounds. I tried searching the web and depending on how I rephrased my words I got various results of different compounds. For instance if I typed polyglycerol ester of fatty acids I got as results a.
Γ T and Γ R can be obtained directly from the character table. Γ 3N 3A 1 A 2 3B 1 2B 2. Γ T A 1 B 1 B 2.
Γ R A 2 B 1 B 2. Γ VIB Γ 3N - Γ T - Γ R 2A 1 B 1. So the three normal modes of vibration for water have the symmetries A 1 A 1 and B 1.
The majority of phosphorus-containing compounds are produced for use as fertilizers. For this purpose phosphate-containing minerals are converted to phosphoric acid. Inorganic phosphorus in the form of the phosphate PO 4 3 is required for all known forms of life.
It plays a major role in biological molecules. For example it forms part of the structural framework of DNA and RNA. Until 1978 lead compounds were an important component of many paints.
Lead was added to paint to promote adhesion corrosion control drying and covering. White lead lead carbonate linseed oil and inorganic pigments were the basic components for paint in the 18th and 19th centuries and continued until the middle of the 20th century. Answer 1 of 18.
It depends on your skills and talents. If math calculus through differential equations is easy for you then physical chemistry will be a piece of cake. Inorganic and organic will require other skills that you may or may not have.
If you truly get electro.