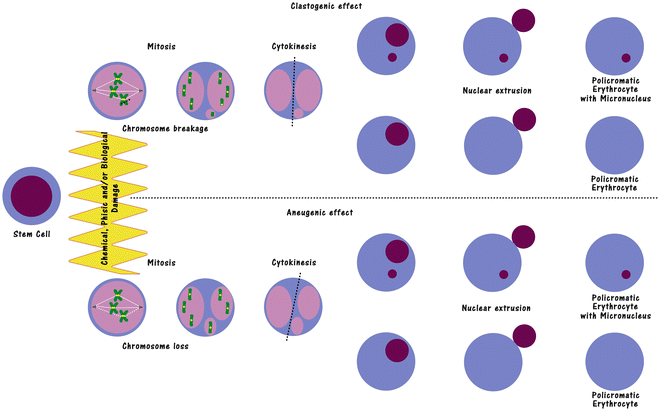
Limited evidence of clastogenicity was obtained in Chinese Hamster ovary cells although this finding was not confirmed in a second in vitro assay utilising Chinese Hamster lung cells nor in vivo assays including a mouse spermatogonia chromosomal aberration assay a mouse micronucleus assay and a rat bone marrow clastogenicity assay. In vivo relevant concentrations of 001 to 01 umolL atorvastatin or mevastatin promote the migration of mature endothelial cells and tube formation.
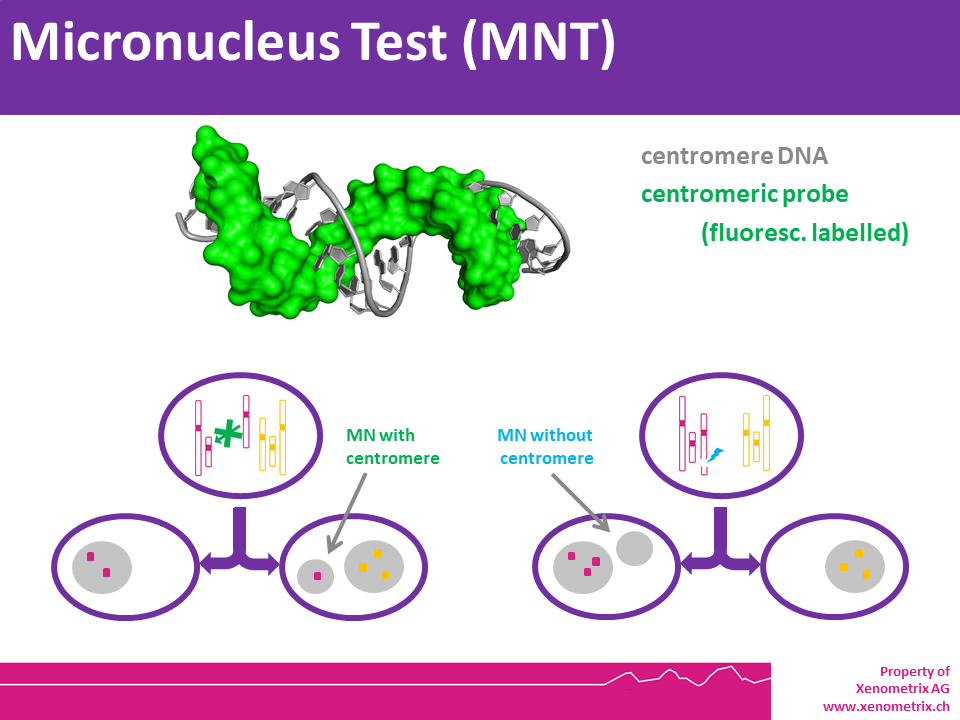
Mometasone furoate did not cause DNA damage in rat liver.
In vivo micronucleus assay presentation. Inhibition potency must always be considered in the context of expected in vivo concentrations of the test compound. Although the criteria for acceptance are project and isoform-specific potent inhibition is considered unfavorable and may preclude the development of a compound. What substrates and positive control inhibitors are used in the cytochrome P450 inhibition assay.
Cyprotexs in vitro toxic hemolysis assay is a sensitive and accurate method for predicting toxic hemolysis of a drug. However it should be noted that hemolysis sometimes occurs when blood is drawn. This should be taken into account when performing the assay.
Also colored drugs that absorb light at the same wavelength at which the assay is measured 540 nm can lead to false positives. Use of cell-based assays as opposed to target enzyme assay in isolation will mimic the in vivo environment when exposed to a drug. Protein binding affinity of a drug molecule governs the available effective concentration of free drug in the system.
Whole-cell treatment in culture media having serum would represent the free and bound drug combination as observed in whole animal. Disease and. The European Journal of Cancer EJC integrates preclinical translational and clinical research in cancer from epidemiology carcinogenesis and biology through to innovations in cancer treatment and patient careThe journal publishes original research reviews previews editorial comments and correspondence.
The EJC is the official journal of the European Organisation for Research and. Data and research on test guidelines including chemical testing and assessment chemical safety animal welfare endocrine disrupters good laboratory practice GLP Mutual Acceptance of Data MAD Because the OECD is committed to chemical safety and animal welfare our new Test Guideline No. 249 identifies chemicals that are toxic to fish by testing cell cultures instead of whole animals.
Drug discovery and development is a time-consuming and costly process. Based on the study by the Tufts Center for the Study of Drug Development 20161 on average it takes more than 10 years and over 26 billion to develop a new drugOne of the biggest challenges for the pharmaceutical research community is therefore to execute an optimal process for drug discovery and. IN VIVO TESTS In vivo test for chromosomal damage using mammalian hematopoietic cells.
Chromosome damage in rodent hematopoietic cells Eg. CARCINOGENICITY ONCOGENICITY STUDIES life-time bioassays carcinogenicity studies are performed on. Drug used for 6 months or frequent intermittent use for chronic diseases.
Presentation by Dr. Scott Auerbach at the NTP Board of Scientific Counselors Meeting on June 16 2015. Society of Toxicology San Diego CA March 2226 2015 Poster.
HTS and SAR Analysis of Chemicals from the Elk River Spill. Maternal and Prenatal Dose Range-Finding Study of 4-Methylcyclohexanemethanol MCHM in Harlan Sprague Dawley Rats. Society of Toxicology New.
In vitro Mouse Lymphoma TK13 -Gene Mutation Assay 14 April 2006 d. In vivo Mammalian Erythrocyte Micronucleus Test15 July 2000 2. Consequently two distinct in vivo rodent mutagenicity assays that are recognized as robust tools for evaluating mutagenicity and for assessing human risk for mutagenicity the Pig-a mutagenicity assay and the Big Blue cII Locus transgenic rodent assay were conducted.
In both assays which were run at doses and durations significantly greater than those being used in the clinic the. Reduction and refinement methods for in vivo testing shall also be encouraged to keep the number of animals used in testing to a minimum. The principles of replacement reduction and refinement of the use of animals shall be taken into account in the design of the test methods in particular when appropriate validated methods become available to replace reduce or refine animal testing.
4-Trifluoromethylaniline 4-TFMA a minor metabolite of teriflunomide was positive in the in vitro bacterial reverse mutation Ames assay the in vitro HPRT assay and the in vitro chromosomal aberration assay in mammalian cells. 4-TFMA was negative in in vivo micronucleus and chromosomal aberration assays. Other types of genotoxic tests resulted in negative findings.
Carisoprodol was not mutagenic in the Ames reverse mutation assay using S. Typhimurium strains with or without metabolizing enzymes and was not clastogenic in an in vivo mouse micronucleus assay of circulating blood cells. Limited evidence of clastogenicity was obtained in Chinese Hamster ovary cells although this finding was not confirmed in a second in vitro assay utilising Chinese Hamster lung cells nor in vivo assays including a mouse spermatogonia chromosomal aberration assay a mouse micronucleus assay and a rat bone marrow clastogenicity assay.
Mometasone furoate did not cause DNA damage in rat liver. The mutagenic potential of lidocaine has been tested in the Ames Salmonella reverse mutation assay an in vitro chromosome aberrations assay in human lymphocytes and in an in vivo mouse micronucleus assay. There was no indication of any mutagenic effect in these studies.
Citalopram was not mutagenic in the in vitro mammalian forward gene mutation assay HPRT in mouse lymphoma cells or in a coupled in vitroin vivo unscheduled DNA synthesis UDS assay in rat liver. It was not clastogenic in the in vitro chromosomal aberration assay in human lymphocytes or in two in vivo mouse micronucleus assays. Long-term animal studies of HUMIRA have not been conducted to evaluate the carcinogenic potential or its effect on fertility.
No clastogenic or mutagenic effects of HUMIRA were observed in the in vivo mouse micronucleus test or the Salmonella-Escherichia coli Ames assay respectively. Genotoxicity studies performed with neomycin and polymyxin B with and without metabolic activation were negative in bacterial Ames test or mammalian cells chromosomal aberration assay in CHO cells. Dexamethasone was clastogenic in vivo in the mouse micronucleus assay at doses in excess of those obtained following topical application.
Genotoxicity studies performed with neomycin and polymyxin B with and without metabolic activation were negative in bacterial Ames test or mammalian cells chromosomal aberration assay in CHO cells. Dexamethasone was clastogenic in vivo in the mouse micronucleus assay at doses in excess of those obtained following topical application. Assay that looks for antibodies in vivo against red blood cells caused by various types of infections drug reactions and autoimmune disorders.
Enzyme-linked immunoabsorbent assay in which the antigens are immobilized in the well of a microtiter plate. Only a single antibody is used in the test. Direct fluorescent antibody DFA.
New test data demonstrate that Allura Red AC neither damages the DNA of individual cells comet assay nor shows other evidence of genotoxicity in-vivo micronucleus test. EFSA experts extrapolated safe dietary levels for dogs and cats from available toxicity studies. They calculated the highest safe dietary concentration of Allura Red AC to be 370 mgkg complete feed for dogs and 308.
A major depressive episode may be the initial presentation of bipolar disorder. It is generally believed though not established in controlled studies that treating such an episode with an antidepressant alone may increase the likelihood of precipitation of a mixedmanic episode in patients at risk for bipolar disorder. Whether any of the symptoms described above represent such a conversion.
Results in the in vivo mouse micronucleus test were also negative. No carcinogenicity studies have been conducted with the combined components of Janumet or Janumet XR. A two-year carcinogenicity study was conducted in rats given oral doses of sitagliptin of 50 150 and 500 mgkgday.
Citalopram was not mutagenic in the in vitro mammalian forward gene mutation assay HPRT in mouse lymphoma cells or in a coupled in vitroin vivo unscheduled DNA synthesis UDS assay in rat liver. It was not clastogenic in the in vitro chromosomal aberration assay in human lymphocytes or in two in vivo mouse micronucleus assays. In vivo relevant concentrations of 001 to 01 umolL atorvastatin or mevastatin promote the migration of mature endothelial cells and tube formation.
Moreover atorvastatin also increases migration and the potency to form vessel structures of circulating endothelial progenitor cells which may contribute to vasculogenesis. In contrast higher concentrations 01 umolL atorvastatin block.