Hydrogen peroxide comes in the dark brown bottle because it breaks down to plain water when exposed to heat light and air. Large quantities of hydrogen are used to hydrogenate oils to form fats for.

Chances are youve probably come into contact with one or both of these chemical compounds at some point.
Hydrogen peroxide properties and uses. The redox properties of hydrogen peroxide depend on pH as acidic conditions exacerbate the power of oxidizing agents and basic conditions the power of reducing agents. As hydrogen peroxide exhibits ambivalent redox properties being simultaneously an oxidizer or a reductant its redox behavior immediately depends on pH. In acidic solutions H 2 O 2 is a powerful oxidizer stronger than.
Hydrogen peroxide is the simplest kind of peroxide. It has an open book structure with O-O spins. Understand Structure Properties Preparation Uses.
Determine the Concentration of H2O2. Amazing Hydrogen Peroxide Uses. Most people associate hydrogen peroxide with its ability to be used to treat minor medical conditions which is why you will find the chemical compound in the pharmaceutical section of your local store.
Here are some common medical uses of a bottle of hydrogen peroxide and a few uses you may never have considered. Hydrogen peroxide is a mild antiseptic used on the skin to prevent infection of minor cuts scrapes and burns. It may also be used as a mouth rinse to help remove mucus or to relieve minor mouth.
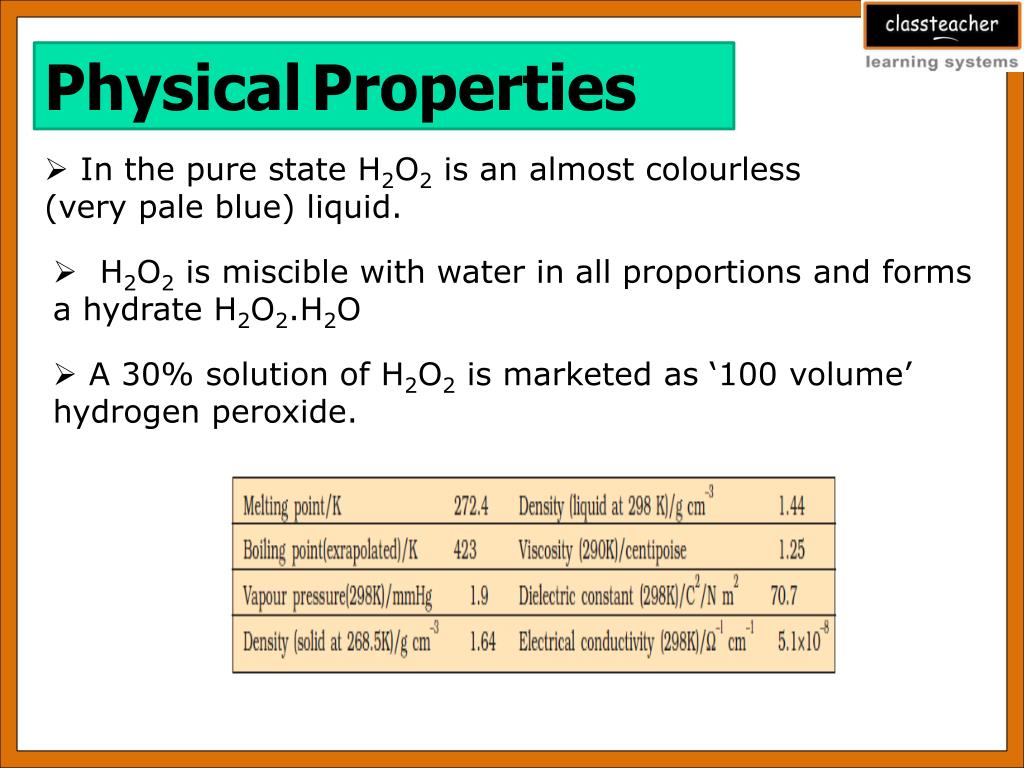
The most important covalent peroxide is hydrogen peroxide H 2 O 2. When pure this syrupy viscous liquid has a pale blue colour although it appears almost colourless. Many of its physical properties resemble those of water.
It has a larger liquid range than water melting submarine. World War IIsystem using oxygen generated by hydrogen peroxide to operate the turbine while submerged. Hydrogen peroxide is a hydrogen-oxygen chemical compound.
Anhydrous hydrogen peroxide is a colourless syrupy liquid that decomposes into oxygen and water very easily. H 2 O 2 hydrogen peroxide is a colorless liquid that is similar to water in several ways. It has physical properties that are very similar to water with the exception that it.
Hydrogen peroxide mixed with sodium is known as oxygen bleach. Add water and the compound releases an oxygen molecule to help it lift mold and stains from the surface of natural materials. Hydrogen also has many other uses.
In the chemical industry it is used to make ammonia for agricultural fertiliser the Haber process and cyclohexane and methanol which are intermediates in the production of plastics and pharmaceuticals. It is also used to remove sulfur from fuels during the oil-refining process. Large quantities of hydrogen are used to hydrogenate oils to form fats for.
Hydrogen peroxide H2O2 CID 784 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. Public health information CDC Research information NIH SARS-CoV-2 data NCBI Prevention and treatment information HHS. This is because of its ability to generate antimicrobial and oxygen properties.
The only difference between peroxide and water is the extra oxygen atom that it contains that breaks down releasing the molecule of water. It is this extra oxygen atom that makes hydrogen peroxide such a useful ingredient. It acts as a supplement of oxygen for your plants and aerates the soil when the oxygen is.
Hydrogen peroxide Reactions Physical Properties an Uses H 2 O 2. Hydrogen peroxide is a very common chemical compound in the laboratory and have lot of uses in home and industrial scale. It is used as a disinfectant in the cleaning of injuries.
In this tutorial we will learn about preparation reactions and other characteristics of hydrogen peroxide. Physical Properties of hydrogen peroxide. Hydrogen peroxide comes in the dark brown bottle because it breaks down to plain water when exposed to heat light and air.
The decomposition isnt harmful but if the fizz is gone when youre beginning cleaning youre just using plain water. Use the bottle within a month or so of opening for best results but know that hydrogen peroxide can still be used for about six months after opening. It plays a significant role in our lives because of its oxidizing properties making H2O2 a very effective anti-fungal anti-viral and anti-bacterial disinfectant agent.
What is Food-Grade Hydrogen Peroxide. Hydrogen peroxide commonly used and found in different forms often comes mixed with harmful chemicals such as phenol acetanilide tetrasodium pyrophosphate and sodium stannate. Hydrogen peroxide has several properties that make it an effective cleaner.
Firstly it produces compounds known as free radicals which attack and. Use hydrogen peroxide to remove the dead skin from your dry cracked heels. Not only will it soften calluses but hydrogen peroxide will also aid the healing process.
How to Treat Cracked Heels. Mix 2 cups of hydrogen peroxide and 2 cups of hot water. Soak your feet in the mixture for 30 minutes.
Rinse and dry feet. Hydrogen peroxide or H202 as it is scientifically known comes in a variety of forms. Depending on the concentration of the mixture the liquid is considered for household use food grade or electrical uses.
In whatever form though hydrogen peroxide is nothing more than oxygen and water combined in a unique ratio to form a germicidal liquid. Tritium hydrogen-3 produced in nuclear reactors is used in the production of hydrogen bombs as an isotopic label in the biosciences and as a source of beta radiation in radioluminescent paint for instrument dials and emergency signage. H 2 is a product of some types of anaerobic metabolism and is produced by several microorganisms usually via.
Hydrogen peroxide possesses antifungal properties that can alleviate the itching and burning caused by foot fungus and athletes foot. All you need to do is combining equal parts of peroxide and water. Then apply the combination to your foot.
Benefits Of Hydrogen Peroxide Treat Mites Infection. Hydrogen peroxide is a wonderful treatment for mite infected skin. You can spray some.
Hydrogen peroxide is a pale blue liquid which appears colorless in a dilute solution and is slightly thicker than water. H2O2 is a weak acid with strong oxidizing properties. This makes it a powerful bleaching agent mostly used for paper and also handy as a disinfectant and as an oxidizer.
Peroxide also has bacteriostatic properties meaning it helps prevent bacteria from growing and dividing and also acts as a sporicide killing potentially infectious fungal spores. However hydrogen peroxide isnt an ideal disinfectant because it also kills fibroblasts which are a type of connective tissue the body uses to help repair wounds. Rubbing alcohol and hydrogen peroxide are two common household cleaners.
Chances are youve probably come into contact with one or both of these chemical compounds at some point. This extra oxygen H2O2 gives hydrogen peroxide its beneficial properties. So the answer to the question Does hydrogen peroxide hurt plants is a resolute no provided the strength is sufficiently diluted.
You can purchase hydrogen peroxide in various potencies. The most commonly available is a 3 solution but they go up to 35. The 3 solution is the type readily available at the.
So hydrogen peroxide has many great uses around the home. Its antimicrobial properties and cleansing action can help to keep surfaces free from viruses and bacteria and it also makes for an effective home remedy for earwax and ear infections. Read these related articles.
The Best Home Remedies For Getting Rid of Ear Infection 2. How to Naturally Get Rid of Clogged Ears 3. The hydrogen peroxide vapor diffuses through the chamber 50 minutes exposes all surfaces of the load to the sterilant and initiates the inactivation of microorganisms.
An electrical field created by a radio frequency is applied to the chamber to create a gas plasma. Microbicidal free radicals eg hydroxyl and hydroperoxyl are generated in the plasma. The excess gas is removed and in.