When hydrogen peroxide exists as pure viscous at liquid state it is colourless. When at solid state hydrogen peroxide is a white crystal.

Also at higher temperature H 2 O 2 may be explosive.
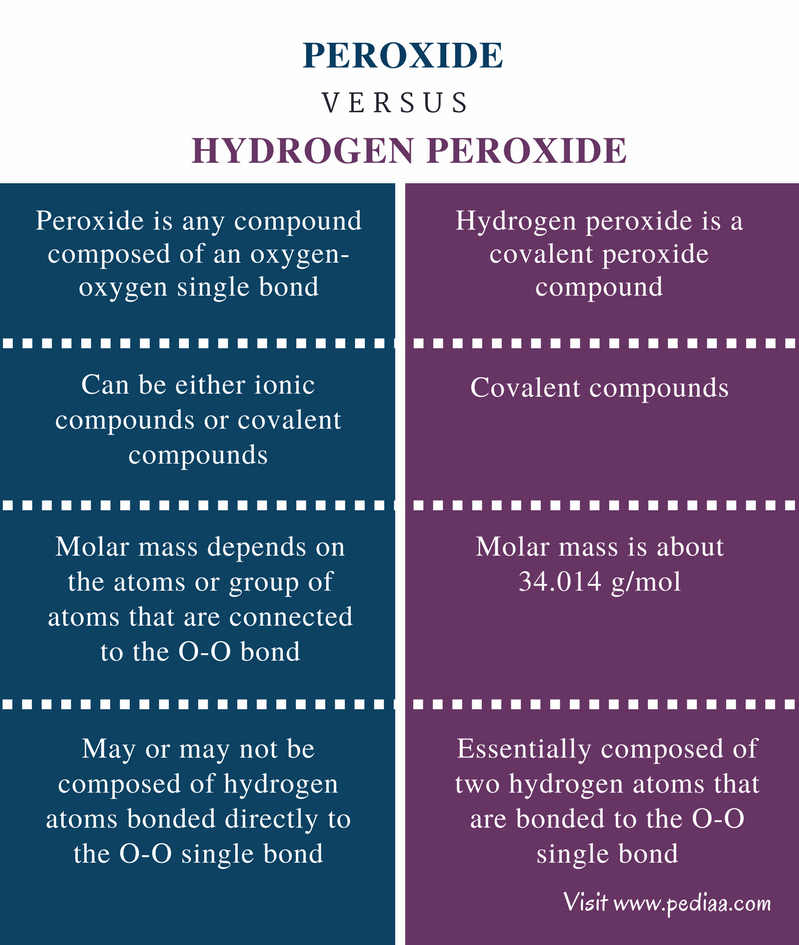
Hydrogen peroxide properties. The redox properties of hydrogen peroxide depend on pH as acidic conditions exacerbate the power of oxidizing agents and basic conditions the power of reducing agents. As hydrogen peroxide exhibits ambivalent redox properties being simultaneously an oxidizer or a reductant its redox behavior immediately depends on pH. In acidic solutions H 2 O 2 is a powerful oxidizer stronger than.
Properties of Hydrogen Peroxide Physical Properties. In the pure state hydrogen peroxide is almost colourless very pale blue liquid. It melts at 2724 K and has a boiling point of 423 K extrapolated.
It is miscible in water in all proportions and forms hydrates. Hydrogen peroxide in both acidic and basic medium acts as an oxidizing as well as a reducing agent. Hydrogen peroxide - urea also called Hyperol artizone urea hydrogen peroxide and UHP is a solid composed of equal amounts of hydrogen peroxide and ureaThis compound is a white crystalline solid which dissolves in water to give free hydrogen peroxide.
Hydrogen peroxide - urea contains solid and water-free hydrogen peroxide which offers a higher stability and better controllability than. Hydrogen peroxide H2O2 CID 784 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more. Public health information CDC Research information NIH SARS-CoV-2 data NCBI Prevention and treatment information HHS.
If youre tired of the itching and burning that comes with athletes foot and foot fungus hydrogen peroxides antifungal properties may be the perfect antidote. Combine equal parts water and peroxide and apply to the foot to see your athletes foot cured. You can also use the mixture on your shower shoes to get rid of foot fungus in its tracks and prevent athletes foot before it.
Physical Properties of hydrogen peroxide. When hydrogen peroxide exists as pure viscous at liquid state it is colourless. When at solid state hydrogen peroxide is a white crystal.
Also at higher temperature H 2 O 2 may be explosive. When exposes to the sunlight hydrogen peroxide decomposes to oxygen gas and water. Has weak acidic characteristics.
Hydrogen peroxide is a mild antiseptic used on the skin to prevent infection of minor cuts scrapes and burns. It may also be used as a mouth rinse to help remove mucus or to relieve minor mouth. The most important covalent peroxide is hydrogen peroxide H 2 O 2.
When pure this syrupy viscous liquid has a pale blue colour although it appears almost colourless. Many of its physical properties resemble those of water. It has a larger liquid range than water melting submarine.
World War IIsystem using oxygen generated by hydrogen peroxide to operate the turbine while submerged. Hydrogen peroxide is an inexpensive household product you probably have on hand right now. When used carefully it can be an effective way to whiten your teeth.
But if used incorrectly in. Hydrogen peroxide has several properties that make it an effective cleaner. Firstly it produces compounds known as free radicals which attack and.
Hydrogen peroxide is a hydrogen-oxygen chemical compound. Anhydrous hydrogen peroxide is a colourless syrupy liquid that decomposes into oxygen and water very easily. H 2 O 2 hydrogen peroxide is a colorless liquid that is similar to water in several ways.
It has physical properties that are very similar to water with the exception that it. Hydrogen peroxide or H202 as it is scientifically known comes in a variety of forms. Depending on the concentration of the mixture the liquid is considered for household use food grade or electrical uses.
In whatever form though hydrogen peroxide is nothing more than oxygen and water combined in a unique ratio to form a germicidal liquid. Its these hydrogen bonds that give water many of its properties. If they didnt exist the boiling point of water would be below -70 degrees Celsius.
Liquid water would not feature on the Earth. Hydrogen was the unwitting discovery of Paracelsus the sixteenth century Swiss alchemist also known as Theophrastus Philippus Aureolus Bombastus von Hohenheim. He found that something flammable.
Hydrogen peroxide is a pale blue liquid which appears colorless in a dilute solution and is slightly thicker than water. H2O2 is a weak acid with strong oxidizing properties. This makes it a powerful bleaching agent mostly used for paper and also handy as a disinfectant and as an oxidizer.
Hydrogen peroxide works to disinfect wounds in several ways. First since its a solution in water it helps rinse away dirt and damaged cells and loosen dried blood while the bubbles help lift away debris. Although the oxygen released by peroxide doesnt kill all types of bacteria some are destroyed.
Peroxide also has bacteriostatic properties meaning it helps prevent bacteria from growing. Hydrogen peroxide comes in the dark brown bottle because it breaks down to plain water when exposed to heat light and air. The decomposition isnt harmful but if the fizz is gone when youre beginning cleaning youre just using plain water.
Use the bottle within a month or so of opening for best results but know that hydrogen peroxide can still be used for about six months after opening. Hydrogen peroxide mixed with sodium is known as oxygen bleach. Add water and the compound releases an oxygen molecule to help it lift mold and stains from the surface of natural materials.
High-strength hydrogen peroxide Hydrogen dioxide Hydrogen peroxide aqueous Hydroperoxide Peroxide Colorless liquid with a slightly sharp odor. The pure compound is a crystalline solid below 12F. Often used in an aqueous solution.
This extra oxygen H2O2 gives hydrogen peroxide its beneficial properties. So the answer to the question Does hydrogen peroxide hurt plants is a resolute no provided the strength is sufficiently diluted. You can purchase hydrogen peroxide in various potencies.
The most commonly available is a 3 solution but they go up to 35. The 3 solution is the type readily available at the. How Plants Can Use Hydrogen Peroxide.
A colorless liquid hydrogen peroxide has a chemical formula of H2O2 which is similar to water. Although hydrogen peroxide is commercially available it cant be considered organic because plants and animals produce the chemical naturally in their tissues and cells. Hydrogen peroxide is an inexpensive natural remedy for a tooth abscess.
Hydrogen peroxide is made up of hydrogen and oxygen. It has antiseptic and antibacterial properties. The brown bottle of 3 hydrogen peroxide from the drugstore or pharmacy is ideal for treating an abscess.
Never use full strength food grade hydrogen peroxide internally or externally. Mix 1 tablespoon of your 3. The hydrogen peroxide vapor diffuses through the chamber 50 minutes exposes all surfaces of the load to the sterilant and initiates the inactivation of microorganisms.
An electrical field created by a radio frequency is applied to the chamber to create a gas plasma. Microbicidal free radicals eg hydroxyl and hydroperoxyl are generated in the plasma. The excess gas is removed and in.
Is Hydrogen Peroxide Effective. Yes hydrogen peroxide has been found to be quite effective as both a cleaning and whitening agent. For cleaning hydrogen peroxide kills bacteria and breaks up plaque in the mouth.
And for whitening hydrogen peroxide is a compound with properties that can break down the molecules within the tooth that cause. Hydrogen peroxide is an antimicrobial agent that you may already have in your medicine cabinet. It is scientifically known as H 2 O 2 and can come in different forms and concentrations.
Most people have a bottle of 3 hydrogen peroxide in their house because it is useful to get rid of stains kill germs in the kitchen get rid of mold and treat toenail fungus.