The choice of the chlorine type to be used often depends on cost on the available storage options and on the pH conditions required. Hydrogen peroxide is versatile it can be used for many applications.
Thus a melting point reflects the thermal energy needed to convert the highly ordered array of molecules in a crystal lattice to the randomness of a.
Hydrogen and chlorine are pure substances or. Chlorine played an important role in the experiments conducted by medieval alchemists which commonly involved the heating of chloride salts like ammonium chloride sal ammoniac and sodium chloride common salt producing various chemical substances containing chlorine such as hydrogen chloride mercuryII chloride corrosive sublimate and hydrochloric acid in the form of aqua regia. Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element.
At standard conditions hydrogen is a gas of diatomic molecules having the formula H 2It is colorless odorless non-toxic and highly combustibleHydrogen is the most abundant chemical substance in the universe constituting roughly 75 of all normal matter. 82 FDA Indirect Additives used in Food Contact Substances. Title 21 of the US.
Code of Federal Regulations 21 CFR 176180. FDA Center for Food Safety and Applied Nutrition CFSAN 83 Food Additive Status. FDA Food Additive Status.
Microcrystalline cellulose - MISC FS Unlisted GRAS. FDA Center for Food. This method produced pure hydrogen peroxide.
Nowadays self-oxidation processes are used to produce hydrogen peroxide. During these processes hydrogen is the raw material. Versatility of hydrogen peroxide.
Hydrogen peroxide is versatile it can be used for many applications. It can be used in all media. Air water waste water and soils.
It is sometimes used combined with other agents to. Common hydrogen has a molecular weight of 201594 g. As a gas it has a density of 0071 gl at 0ºC and 1 atm.
Its relative density compared with that of the air is 00695. Hydrogen is the most flammable of all the known substances. Hydrogen is slightly more soluble in organic solvents than in water.
Many metals absorb hydrogen. Pure chlorine is seldom used for water treatment. The three most common chlorine-containing substances used in water treatment are chlorine gas sodium hypochlorite and calcium hypochlorite.
The choice of the chlorine type to be used often depends on cost on the available storage options and on the pH conditions required. Chlorination affects pH and pH affects resultsa fact that is. However pure chlorine gas can be directly combined with hydrogen to produce hydrogen chloride directly in the presence of UV light.
This is a highly exothermic reaction and rarely used commercially to produce HCl. Hydrogen is used to turn unsaturated fats to saturated oils and fats. Food industries for instance use hydrogen to make hydrogenated vegetable.
Chlorine is a commonly used household cleaner and disinfectant. Chlorine is a potent irritant to the eyes the upper respiratory tract and lungs. Chronic long-term exposure to chlorine gas in workers has resulted in respiratory effects including eye and throat irritation and airflow obstruction.
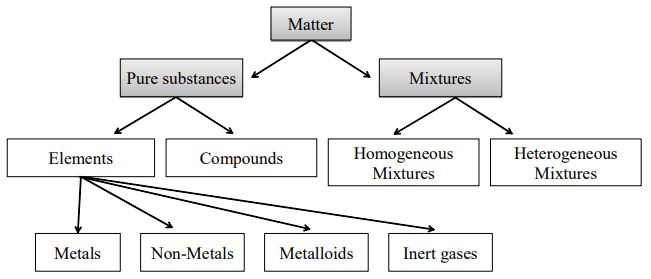
No information is available on the carcinogenic effects of chlorine in humans from inhalation. Mixtures can always be separated again into component pure substances because bonding among the atoms of the constituent substances does not occur in a mixture. Whereas a compound may have very different properties from the elements that compose it in mixtures the substances keep their individual properties.
For example sodium is a soft shiny metal and chlorine is a. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structureThe chemical symbol for Hydrogen is H. With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table.
Its monatomic form H is the most abundant chemical substance in the Universe constituting roughly 75 of all baryonic mass. Hydrogen peroxide decomposes into water and oxygen upon heating or in the presence of numerous substances particularly salts of such metals as iron copper manganese nickel or chromium. It combines with many compounds to form crystalline solids useful as mild oxidizing agents.
The best-known of these is sodium perborate NaBO 2 H 2 O 2 3H 2 O or NaBO 3 4H 2 O used in laundry. The chlorination process involves adding chlorine to water but the chlorinating product does not necessarily have to be pure chlorine. Chlorination can also be carried out using chlorine-containing substances.
Depending on the pH conditions required and the available storage options different chlorine-containing substances can be used. The three most common types of chlorine used. The substances have fixed boiling and melting points.
A pure substance usually participates in a chemical reaction to form predictable products. Examples of Pure Substances. All elements are mostly pure substances.
A few of them include gold copper oxygen chlorine diamond etc. Compounds such as water salt or crystals baking soda amongst. An element in science or specifically chemistry can be defined as any substance that cannot be separated into different substances by ordinary chemical methods Each element is made of a specific and unique type of atom.
Everything in the universe is made of one or more elements. Pure water is a compound made from two elements - hydrogen and oxygen. The ratio of hydrogen to oxygen in water is always 21.
Each molecule of water contains two hydrogen atoms bonded to a single oxygen atom. Pure table salt is a compound made from two elements - sodium and chlorine. The ratio of sodium ions to chloride ions in sodium chloride is always 11.
Hydrogen Peroxide Vinegar Katja Cho You may have heard that you should spray fruits or countertops with alternating mists of hydrogen peroxide and vinegar. Elements are Mercury Iron Diamond Nitrogen Graphite Hydrogen Oxygen and chlorine. State three reasons why you think air is a mixture and water is a compound.
Air is a mixture because. It contains two or more pure substances. By physical processes it can be separated into simpler substance.
Its composition is not fixed. Water is a compound because. The millions of different chemical compounds that make up everything on Earth are composed of 118 elements that bond together in different ways.
This module explores two common types of chemical bonds. The module presents chemical bonding on a sliding scale from pure covalent to pure ionic depending on differences in the electronegativity of the bonding atoms. Not all substances are made up of distinct molecular units.
Sodium chloride common table salt for example consists of sodium ions and chlorine ions arranged in a lattice so that each sodium ion is surrounded by six equidistant chlorine ions and each chlorine ion. The melting and boiling points of pure substances reflect these intermolecular forces and are commonly used for identification. Of these two the boiling point is considered the most representative measure of general intermolecular attractions.
Thus a melting point reflects the thermal energy needed to convert the highly ordered array of molecules in a crystal lattice to the randomness of a. Produce Hydrogen Chloride and Chloric Acid gases. Store in tightly closed containers in a cool well-ventilated area away from COMBUSTIBLES.
Chlorine Dioxide is sensitive to SHOCK and FRICTION and unstable in LIGHT and SUNLIGHT. Transportation of pure Chlorine Dioxide is FORBIDDEN by DOT. Keep frozen when not in use.
Store in tightly. For example the boiling point of pure water at standard atmospheric pressure or sea level is 100C 212F while at 10000 feet 3048m it is 9039 C 1947F. This decrease will affect the time it takes to cook anything in water to the extent that any food that requires five minutes to prepare at sea level will take around 20 minutes at 3km 10000 feet.
In theory you could also. The molecular weight of a pure compound is determined from its chemical formula and the atomic weights of its elements. The atomic weights of the elements found in organic substances are C 12011 H 1008 S 32065 O 15999 and N 14007.
The molecular weight of ethanol C 2 H 5 OH To calculate the molecular weight of ethanol the molecular weight of each atom in the. Ozones odor is sharp reminiscent of chlorine and detectable by many people at concentrations of as little as 10 parts per billion in air. In standard conditions ozone is a pale blue gas that condenses at progressively cryogenic temperatures to a dark blue liquid and finally a violet-black solid.
Ozone is a powerful oxidant far more so than dioxygen and has many industrial and consumer. On 1 May 2014 a paper published in Phys. Khuyagbaatar and others states the superheavy element with atomic number Z 117 ununseptium was produced as an evaporation residue in the 48 Ca and 249 Bk fusion reaction at the gas-filled recoil separator TASCA at GSI Darmstadt Germany.
The radioactive decay of evaporation residues and their α-decay products was studied using a.