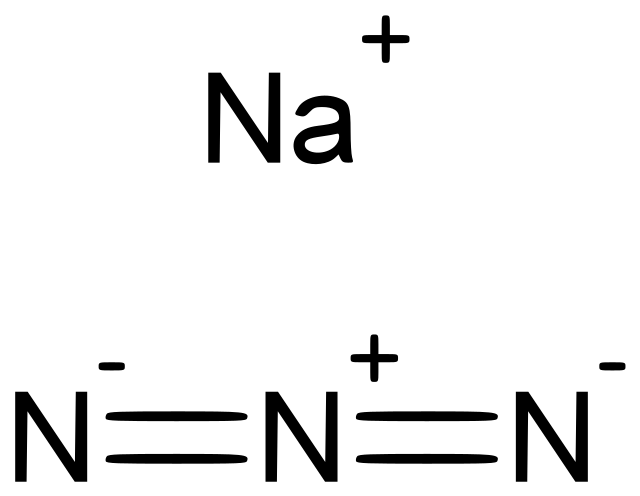
A micro-dialysis unit with a cut off at 14000 daltons will retain the antibody as the azide diffuses out. The azide-alkyne Huisgen cycloaddition is a 13-dipolar cycloaddition between an azide and a terminal or internal alkyne to give a 123-triazole.

Tellurite sodium azide or thallium acetate at concentrations of 01 - 05 gl will inhibit the growth of Gram-negative bacteria.
How much sodium azide to use. Sodium azide in a sample was acidified and the azide was converted to the volatile hydrazoic acid which was trapped in 25 mM sodium hydroxide solution. Determination was performed by isocratic ion chromatography using suppressed conductivity detection. Calibration curves were linear for 05 to 20 ugmL sodium azide and the detection limit was 005 ugmL.
Recoveries of sodium azide from. Metallic sodium is used mainly for the production of sodium borohydride sodium azide indigo and triphenylphosphine. A once-common use was the making of tetraethyllead and titanium metal.
Because of the move away from TEL and new titanium production methods the production of sodium declined after 1970. Sodium is also used as an alloying metal an anti-scaling agent and as a reducing agent. Sodium azide NaN 3 can decompose at 300 o C to produce sodium metal Na and nitrogen gas N 2.
The signal from the deceleration sensor ignites the gas-generator mixture by an electrical impulse creating the high-temperature condition necessary for NaN 3 to decompose. The nitrogen gas that is generated then fills the airbag. The purpose of the KNO 3 and SiO 2 is to remove the sodium metal.
The data on the nature a death from Sodium Azide is relatively young. Yes there is little hard science at the moment but watch this space because as time goes by there will be. Any alleged sloppiness in the Sodium Azide data is more down to the need for that data to be gathered clandestinely.
Being present at a persons death is still a legal grey area. Utmost caution is required. The azide-alkyne Huisgen cycloaddition is a 13-dipolar cycloaddition between an azide and a terminal or internal alkyne to give a 123-triazole.
Rolf Huisgen was the first to understand the scope of this organic reactionAmerican chemist Karl Barry Sharpless has referred to this cycloaddition as the cream of the crop of click chemistry and the premier example of a click reaction. One obvious use of this calculator is to find how much alcohol there is in a particular quantity of drink. Knowing the AbV or proof of the liquid and how much of it there is in litres pints bottles etc it is only a matter of putting those two pieces of information in making sure the units of the measure are correct and clicking on Calculate It to get the answer at the bottom.
Sodium azide can be removed from antibody solutions by dialysis or gel filtration. The molecular weight of IgG is 150000 daltons IgM is 600000. The molecular weight of sodium azide is 65 daltons.
A micro-dialysis unit with a cut off at 14000 daltons will retain the antibody as the azide diffuses out. In a beaker on a magnetic stirrer kept at 4C use at least a liter of cold PBS per mL. Sodium nitrite - PRES REG 100 ppm to 200 ppm - In loin muscle of smoked chub - 172177.
REG 10 ppm 0001 sodium nitrite - Alone as color fixative in smoked cured tuna -172175. REG 20 ppm - In cnd pet food containing meat. PS by USDA - As a color fixative and preservative wi or without sodium or potassium nitrate in the curing of red meat.
Metallic sodium is used mainly for the production of sodium borohydride sodium azide indigo and triphenylphosphine. Employed only in rather specialized applications only about 100000 tonnes of metallic sodium are produced annually. Sodium is now produced commercially through the electrolysis of molten sodium chloride.
Protons and Neutrons in Sodium. Sodium is a chemical element with. Sodium azide NaN3 which during a colli-sion decompose rapidly to give nitrogen gas and sodium metal.
Potassium nitrate and sil-icon dioxide are added to remove the sodium metal by converting it into a harmless mate-rial. First the sodium reacts with potassium nitrate to produce potassium oxide K2O sodium oxide Na2O and additional nitro-gen gas. The metal oxides K2O and Na2O react.
In order to make 100 mL of a 17 sodium azide solution you would need to weigh out 17 grams of sodium azide and then add water until the final volume is 100 mL. You can make use of this equation in another way. Say youre told that the solution you will be using has 45 grams of magnesium acetate and the total volume is 245 mL.
What is the concentration of this solution in wv percent. Also if nitrite salts such as sodium nitrite or potassium nitrite become unavailable you can use nitrate salts such as sodium nitrate or potassium nitrate referred to as saltpeter or saltpetre because nitrate reduces to nitrite and nitric oxide in vivo. Or use Barbicide which contains isopropyl alcohol dimethyl benzyl ammonioum chloride and sodium nitrite Saha Kane Dargin Nghiem.
Sodium Amide Sodamide NaNH 2 A Strong Base For The Deprotonation Of Terminal Alkynes Among Other Uses. In a blatant plug for the Reagent Guide each Friday I profile a different reagent that is commonly encountered in Org 1 Org 2. Version 12 just got released this week with a host of corrections and a new page index.
Add 200mg of sodium azide contents of 1 eppendorf vial Add 03 ml Triton-X Be sure to use protective clothing and a mask when handling sodium azide under hood. 1 Paraformaldehyde 2 Glutaraldehyde 02M Stock Phosphate Buffer pH 74 500ml Paraformaldehyde 10g Glutaraldehyde 25 in water 80ml Distilledwater QS to 1000ml Dissolve the paraformaldehyde in about 400 ml of water. Use designated area with a sign.
Toxic Compounds Use Area Eye or skin damage Pressurized apparatus CSL 34 lacerations due to shrapnel Chemical fume hood with sash lowered as much as possible Lexan or blast resistant shield SOP Research-specific training and EHS training Safety glasses or chemical splash goggles. Sodium azide affects spectrophotometric absorbance readings between 220 and 280 nm but has no effect on downstream applications such as RT-PCR. Should you wish to determine the purity of the eluted RNA elution with RNase-free water instead of Buffer AVE is recommended.
Cellular DNA contamination The QIAamp Viral RNA Mini Kit is not designed to separate viral RNA from cellular DNA. Wash cells with PBS sodium azide Tris and protein free. Resuspend cells at 10 x 106 cells100 µl of PBS.
Be sure that the PBS does not contain sodium azide Tris or any other protein. Add 1 µl of Zombie dye to 100 µl of cells. To minimize background staining of live cells titrate the dye for optimal performance.
Different cell types can have a wide degree of variability. The only known use of VX is as a chemical warfare agent. By participating in the United Nations International Chemical Weapons Convention treaty the United States agreed to destroy its stockpile of aging chemical weapons.
How people can be exposed to VX. Following release of VX into the air people can be exposed through skin contact eye contact or inhalation breathing in the VX mist. The edible parts of these plants contain much lower amounts of these chemicals.
Cyanide is contained in cigarette smoke and the combustion products of synthetic materials such as plastics. Combustion products are substances given off when things burn. In manufacturing cyanide is used to make paper textiles and plastics.
It is present in the chemicals used to develop photographs. 2 Oral H300. 1 Dermal H310.
STORAGE AND STABILITY. Store kit between 356-86F 2-30C. Ensure all test components are at.
Sodium azide and 01 gelatin. Also available as TransCruz reagent for Gel Supershift and ChIP applications sc-47778 X 200 µg01 ml. β-Actin C4 is available conjugated to agarose sc-47778 AC 500 µg025 ml agarose in 1 ml for IP.
To HRP sc-47778 HRP 200 µgml for WB IHCP and ELISA. To either phycoerythrin sc-47778 PE fluorescein sc-47778 FITC Alexa Fluor 488 sc. Dissolve 500 g NaOH and 135 g NaI in reagent water.
Dilute to 1 L. Add 10 g NaN3 dissolved in 40 ml reagent water. This reagent should not give a color with starch solution when diluted and acidified.
Concentrated Sulfuric acid. Standard sodium thiosulfate titrant 00250N. Prepare an emulsion of 5 g soluble starch.
Including but not limited to Staphene sodium hydroxide sodium hypochlorite and sodium azide are kept in the Immunology Division University of Washington Medical Center UWMC. DATA SYSTEM MANAGEMENT. Each shipment of specimens received from the NHANES mobile unit arrives with a corresponding transmittal sheet and an electronic version of the shippingresulting file.
The use of sodium nitrite NaNO 2 or other nitrites in the presence of secondary or tertiary amines is a potential cause of nitrosamine formation. Secondary amines can be present in reagents and solvents as impurities or degradants. They may also be part of reagents solvents APIs their degradants and precursor structures.
For example amide solvents can degrade to secondary amines which. Tellurite sodium azide or thallium acetate at concentrations of 01 - 05 gl will inhibit the growth of Gram-negative bacteria. Media supplemented with penicillin 5-50 unitsml or crystal violet 2 mgl will inhibit the growth of Gram-positive bacteria.
Tellurite agar therefore is used to select for Gram-positive organisms and nutrient agar supplemented with penicillin can be used to.