HYGIENE MEASURES DO NOT SMOKE IN WORK AREA. When was it introduced into fashion.
Ethanolamines ETHYLENE OXIDE 189 which are used in textile finishing cosmetics soaps detergents and natural gas purification.
How is ethylene glycol made. Ethylene glycol poisoning is poisoning caused by drinking ethylene glycol. Early symptoms include intoxication. Laboratories do not have the ability to perform this blood test and in the absence of this test the diagnosis must be made based on the presentation of the person.
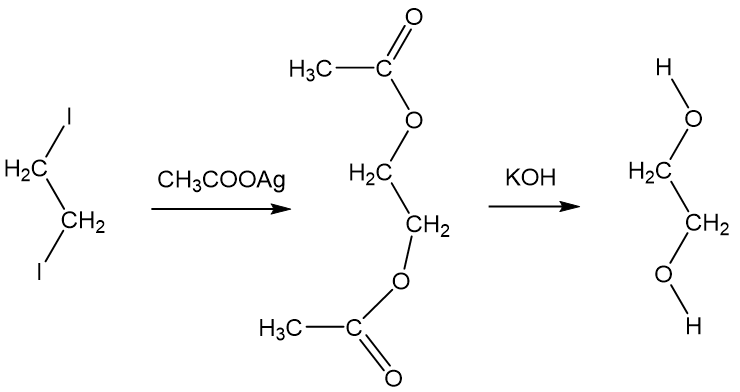
In this situation a helpful test to diagnose poisoning is the measurement of the osmolal gap. The person serum. Mono ethylene glycol also known as MEG EG 12-ethanediol or 12-Dihydroxyethane is an organic compound with the formula C 2 H 6 O 2.
It is a slightly viscous liquid with a clear colourless appearance and a sweet taste that emits virtually no odour. Its miscible with water alcohols and many other organic compounds and is primarily used in the industry for manufacturing polyester. Ethylene glycol is a colorless odorless sweet-tasting chemical.
It is poisonous if swallowed. Ethylene glycol may be swallowed accidentally or it may be taken deliberately in a suicide attempt or as a substitute for drinking alcohol ethanol. Most ethylene glycol poisonings occur due to the ingestion of antifreeze.
This article is for information only. DO NOT use it to treat or manage an. Ethylene glycol is a solvent found in products ranging from antifreeze fluid and de-icing solutions to carpet and fabric cleaners3 4 According to results from animal studies4 the ingested.
A definitive diagnosis of EG poisoning is made with a serum ethylene glycol level. However this is rarely immediately available as almost all hospitals send this test to a reference lab often to an out-of-state laboratory. We are aware of only 4 hospital laboratories in California that can perform this test on a stat basis.
As a result a level drawn at a given hospital can take up to 8-9. Ethylene glycol ethers are part of brake fluids detergents solvents lacquers and paints. Other products of ethylene oxide.
Ethanolamines are used in the manufacture of soap and detergents and for purification of natural gas. Ethoxylates are reaction products of ethylene oxide with higher alcohols acids or amines. They are used in the manufacture of detergents surfactants emulsifiers and.
Ethylene glycol C2H6O2 is a toxic alcohol that is found in various household and industrial agents. The term toxic alcohols is a collective term that includes methanol ethylene glycol and isopropyl alcohol. Ethylene glycol exposure can be extremely dangerous with significant morbidity and mortality if left untreated.
Ethylene glycol is a colorless sweet-tasting liquid most commonly. Ethylene glycol is widely used as antifreeze in automobile cooling systems and in the manufacture of human-made fibres low-freezing explosives and brake fluid. Ethylene glycol and some of its derivatives are mildly toxic.
Propylene glycol also called 12-propanediol resembles ethylene glycol in its physical properties. Mono Ethylene Glycol WELmgm352 mgm3mgm3104 mgm3 PROTECTIVE EQUIPMENT ENGINEERING MEASURES Well-ventilated area. HAND PROTECTION Use protective gloves made of.
Rubber neoprene or PVC. EYE PROTECTION If risk of splashing wear safety goggles or face shield. HYGIENE MEASURES DO NOT SMOKE IN WORK AREA.
Promptly remove any clothing that becomes contaminated. Water is used to cool ethylene glycol in a 48-ft-long double pipe heat exchanger made of 4-std and 2-std both type M copper tubing. The water inlet temperature is 60F and the ethylene glycol inlet temperature is 200F.
The flow rate of the ethylene glycol is 20 lbms while that for the water is 30 lbms. Procedure developed at the Forensic Chemistry Center to examine toothpaste for the presence of Diethylene Glycol and Ethylene Glycol at levels of 500 µgg and above. This method for Diethylene.
87121 Poly ethylene glycol and poly ethylene oxide. PEG and PEO are currently FDA licensed and are used for several tissue engineering applications. Both PEG and PEO have chemical similarities and can be photo-cross-linked by modifying the polymer chain end with acrylates or methacrylates Tan and Marra 2010.
Though many biocompatible polymers can produce hydrogels by chemical. For instance a solution of 10 ethylene glycol freezes at -34 C 259 F 30 ethylene glycol freezes at -137 C 73 F and 60 ethylene glycol freezes at -528 C -63 F. The freezing point of a 6040 ethylene glycolwater mixture is much lower than that of either pure ethylene glycol or pure water.
Mixtures of propylene glycol with water follow a similar pattern with a 6040 mixture of. The basic building blocks of PET are ethylene glycol and terephthalic acid which are combined to form a polymer chain. The resulting spaghetti-like strands of PET are extruded quickly cooled and cut into small pellets.
The resin pellets are then heated to a molten liquid that can be easily extruded or molded into items of practically any shape. PET was first synthesized in North America in. It is made by mixing ethylene glycol and terephthalic acid.
That all sounds extremely scientific but basically polyester is a kind of plastic. When was it introduced into fashion. First invented in 1941 by British chemists John Rex Whinfield and James Tennant Dickson it became increasingly popular in the 1970s thanks to the way it was advertised.
A miracle fiber that can be. Butyl glycol also known as BG 2-butoxyethanol glycol monobutyl ether and ethylene glycol monobutyl ether butyl cellosolve butoxyethanol is a clear colourless oily liquid with a unique sweet yet mild odour and has the formula C 6 H 14 O 2. It is a butyl ether of ethylene glycol and is miscible with water and common organic solvents.
It has been produced industrially for over half a. MolaLity 1074 m Molarity 676 M mole fraction 0162 40 ethylene glycol means mass of ethylene glycol 40 g mass of water solvent 60 g 0060 kg mass of solution 100 g 1. Molality Molar mass of ethylene glycol 6207 gmL no.
Of moles 4 g6207 gmol-1 06444 mol Molality no. Of molesmass of solvent in kg Molality 06444 mol0060 kg 107 m. Ethylene is the starting material for the preparation of a number of two-carbon compounds including ethanol industrial alcohol ethylene oxide converted to ethylene glycol for antifreeze and polyester fibres and films acetaldehyde converted to acetic acid and.
The raw materials used to produce polyethylene glycol are by-products from petroleum refining and can also be derived from natural gas or coal. These are non-renewable sources. Synthesizing PEG compounds can follow multiple different routes depending on the desired end-product.
However the process always involves ethylene oxide. Polyetheylene glycol compounds are listed on product labels. Polyethylene glycol PEG is a polyether compound that has a variety of uses and appearances depending on its molecular weight.
Polyethylene Glycol is made by polymerising ethylene glycol the main ingredient in antifreeze solutions and has a strong presence in the medical industry. Ethylene oxide is a flammable gas with a somewhat sweet odor. It dissolves easily in waterEthylene oxide is a man-made chemical that is used primarily to make ethylene glycol a chemical used to make antifreeze and polyester.
A small amount less than 1 is used to control insects in some stored agricultural products and a very small amount is used in hospitals to sterilize medical. Ethylene oxide is produced from ethylene majorly by direct oxidation. Ethylene is primarily produced from petrochemical-based raw materials such as naptha ethane and propane which are obtained from the distillation of crude oil.
The market is segmented on the basis of derivatives end-user industry and geography. By derivatives the market is segmented into ethylene glycols ethoxylates. Ethylene glycol ethers which are frequently a component of brake fluids detergents and solvents paints and lacquers and are used to treat natural and refinery gas.
Ethanolamines ETHYLENE OXIDE 189 which are used in textile finishing cosmetics soaps detergents and natural gas purification. And ethoxylation products of fatty alcohols fatty amines alkyl phenols cellulose and poly. Polyethylene Glycol is essentially created from ethylene oxide a petrochemical.
For anyone wanting to stay clear of harmful chemicals avoiding petrochemicals is a must. On top of that most PEG ingredients are contaminated with carcinogens as you indicated unless a. These PEF-based materials show tailor-made properties with good ductility.
Recently our group developed a cascade polycondensation. Are assigned to the protons from ethylene glycol units in PEF and methylene oxide units in PTMO segments while those at 721 ppm peak c to the aromatic protons from furan rings respectively. The integral value for each peak is summarized in Table S1.