
Alkyl C 10-C 20 dimethylbenzyl ammonium chloride. Hectorite modified by reaction with a mixture of benzyl methyl dialkyl ammonium chloride and dimethyl dialkyl ammonium chloride where the alkyl groups are derived from hydrogenated tallow CAS Reg.
ALCOHOLS PHENOLS AND ETHERS.
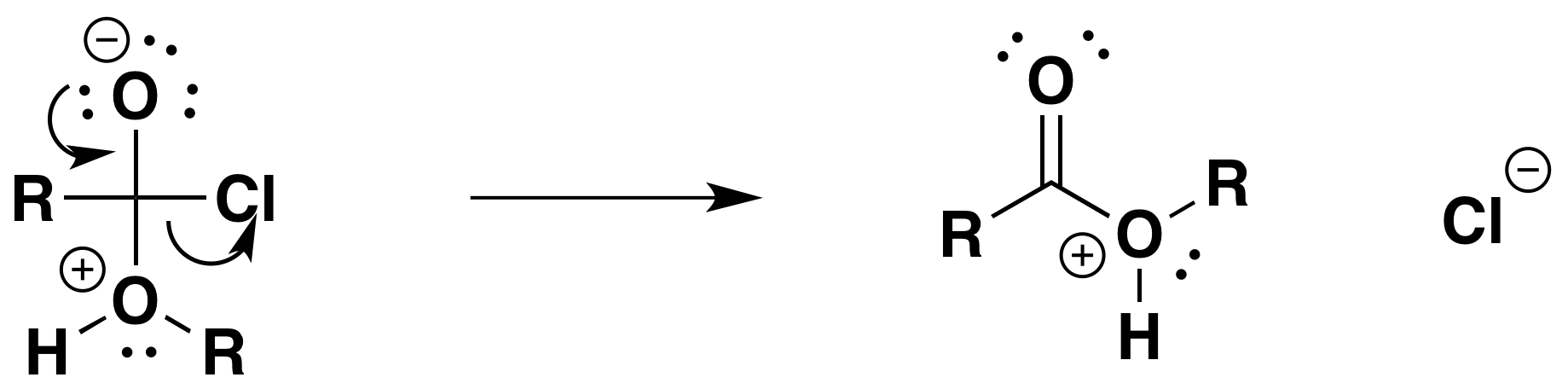
How does benzoyl chloride react with alcohols. Benzoyl chloride is produced by the partial hydrolysis of benzotrichloride. C 6 H 5 CCl 3 H 2 O C 6 H 5 COCl 2 HCl. Similarly benzotrichlorides react with carboxylic acids to the acid chloride.
This conversion is practiced for the reaction of 14-bistrichloromethyl benzene to give terephthaloyl chloride. C 6 H 4 CCl 3 2 C 6 H 4 CO 2 H 2 2 C 6 H 4 COCl 2 2 HCl. The phenoxide ion reacts more rapidly with benzoyl chloride than the original phenol does but even so you have to shake it with benzoyl chloride for about 15 minutes.
Solid phenyl benzoate is formed. Making esters using acid anhydrides. This reaction can again be used to make esters from both alcohols and phenols.
The reactions are slower than. The reaction between ethanoyl chloride and phenol is similar to the ethanol reaction although not so vigorous. Phenyl ethanoate is formed together with hydrogen chloride gas.
Improving the reactions between phenols and some less reactive acyl chlorides. Benzoyl chloride has the formula C 6 H 5 COCl. The -COCl group is attached directly to a.
Benzoyl chloride 2 mL is added in small quantities at a time and the mixture shaken vigorously with occasional cooling under the tap or in ice-water. After 15 min the solid benzoate separates out. The solution should be alkaline at the end of the reaction.
If not alkaline or if the product is oily add a solid pellet of sodium hydroxide and shake again. Collect the benzoate wash. Thionyl chloride is an inorganic compound with the chemical formula S O Cl 2It is a moderately volatile colourless liquid with an unpleasant acrid odour.
Thionyl chloride is primarily used as a chlorinating reagent with approximately 45000 tonnes 50000 short tons per year being produced during the early 1990s but is occasionally also used as a solvent. Benzoyl Peroxide is a peroxide with antibacterial irritant keratolytic comedolytic and anti-inflammatory activity. Upon topical application benzoyl peroxide decomposes to release oxygen which is lethal to the bacteria Proprionibacterium acnes.
Due to its irritant effect benzoyl peroxide increases turnover rate of epithelial cells thereby peeling the skin and promoting the resolution of. It was first prepared by French chemist Charles Frederic Gerhardt in 1852 through heating potassium acetate with benzoyl chloride. Acetic anhydride is prepared by carbonylation of methyl acetate 3.
CH 3 CO 2 CH 3 CO CH 3 CO 2 O. It is also prepared by another process that was developed by Wacker Chemie in 1922. In this method acetic anhydride is prepared by reaction of ketene.
Benzoyl chloride reacts with water to produce benzoic acid which is a white precipitate. Amine can make hydrogen bonds with water. Amine which have less molecular mass are soluble in water.
But solubility is low when alkyl group is large. Methanamine methylamine ethanamine propylamine are. Primary and secondary alcohols require catalysts such as anhydrous zinc chloride.
Tertiary alcohols do not require such catalysts as they are more reactive. 3 Butyronitrile can be prepared by heating propyl chloride with KCN. CH 3-CH 2-CH 2-Cl KCN CH 3-CH 2-CH 2-CN.
Vinyl chloride is primarily used to make polyvinyl chloride to manufacture plastics. Exposure to this substance affects the central and peripheral nervous system and causes liver damage. Prolonged exposure to vinyl chloride can cause a set of symptoms that is characterized by Raynauds phenomenon joint and muscle pain and scleroderma-like skin changes.
Vinyl chloride is a known human. Chlorobenzene reacts with Mg in dry ether to give a compound A which further reacts with ethanol to yield A phenol B benzene C eth. Write chemical reaction of aniline with benzoyl chloride and write the name of the product obtained.
A tertiary amine does not react with benzene suiphonyl chloride and remains insoluble in aqueous KOH. However on acidification with dilute HCI it gives a clear solution due to the formation of the ammonium salt. Ii Reaction with nitrous acid.
All the three types of amines aliphatic. Ii Phenol and benzoyl chloride in the presence of pyridine iii Phenol and benzoyl chloride in the presence of ZnCl2 iv Phenol and benzaldehyde in the presence of palladium. Option ii is the answer.
The reagent which does not react with both acetone and benzaldehyde. I Sodium hydrogen sulphite ii Phenyl hydrazine. ALCOHOLS PHENOLS AND ETHERS.
Phthalimide does not react with chlorobenzene due to large size of phenyl groupC 140Assertion. Acetylation of aniline enhances the tendency to undergo electrophilic substitution. Acetylation reduces the activation of the benzene ring by the NH2 group since it can undergo resonance with both benzene ring and Acetyl group.
Please use this book to increase your knowledge for the laboratory pratictioner. Alkyl C 10-C 20 dimethylbenzyl ammonium chloride. N-AlkylC 12 C 14 C 16 or C 18 dimethyl ethylbenzyl ammonium cyclohexylsulfamate.
For use as preservative only. Alkyl ketene dimers as described in 176120 of this chapter. Alkyl C 7-C 12 naphthalene.
Alpha Olefin sulfonate alkyl group is in the range of C 10-C 18 with not less than 50 percent C 14-C 16 ammonium calcium. This problem does not arise to the same degree in electrophilic additions to alkenes because alkenes are so much more reactive than arenes that the reagents employed eg ceBr_2 ceCl_2 ceHCl ceHOCl ceHOBr ceH_3Ooplus themselves are sufficiently electrophilic to react with alkenes without the aid of a catalyst. In fact conditions that lead.
Hectorite modified by reaction with a mixture of benzyl methyl dialkyl ammonium chloride and dimethyl dialkyl ammonium chloride where the alkyl groups are derived from hydrogenated tallow CAS Reg. For use as a rheological agent only in coatings intended to contact dry food under repeated-use conditions. Column C Sulphonation of aniline.
Caused Bhopal tragedy in 1984. Gives nitrolic acid with HNO2. Can not be used to prepare 2 and 3 amines.
Benzylamine Gives orange dye with diazonium chloride. Does not react with CHCl3KOH. Gives silver mirror with Tollens reagent.
Prussic acid Match the Column Ans. Column A Column B. 6 The additive is manufactured from hops by an initial extraction and fractionation using one or more of the solvents listed in paragraph b5 of this section followed by.
Hydrogenation using palladium as a catalyst in methyl alcohol ethyl alcohol or isopropyl alcohol acidified with hydrochloric or sulfuric acid. Oxidation with peracetic acid. Isomerization by calcium chloride or.
For use only as an adjuvant to control pulp absorbency and pitch content in the manufacture of paper and paperboard prior to the sheet forming operation. Bismethoxymethyltetrakis-octadecyloxy-methylmelamine resins having a 58-65 percent nitrogen content CAS Reg. 68412-27-1 For use only under the following.
Primary alcohols react readily at 2550C while the secondary and tertiary alcohols are about 03 and 0005 times less reactive than the primary ones respectively. Figure 312 presents some commercially important polyols. Figure 312 Some important polyols.
The reaction between a hydroxyl group and an isocyanate is catalyzed by mild and strong bases by many metals and by acids. Product ranges are ketones estersalcohols aldehydes ethers and lactones. We have the most comprehensive range of C3-C15 aliphatic ketones aromatic ketones and high purity electronic chemicals providing to many industries related to our daily life.
Phone Kunshan Odowell 86-512-55380008. Fax Kunshan Odowell. Generic Name Doxycycline DrugBank Accession Number DB00254 Background.
Doxycycline is a broad-spectrum antibiotic synthetically derived from oxytetracycline LabelThis drug is a second-generation tetracycline exhibiting lesser toxicity than first-generation tetracyclines 7Doxycycline may be used to treat a wide range of bacterial infections depending on the results of antibiotic.