The moles of water produced in the neutralization are calculated using the volume and concentration molarity of the acid and base solutions. In an experiment 12 g of sodium hydroxide pellets NaOH s were dissolved in 100 mL of water at 25C.

Get Info Go.
Heat of solution for naoh. Molar heat of solution or molar enthalpy of solution is the energy released or absorbed per mole of solute being dissolved in solvent. Heat of solution enthalpy of solution has the symbol 1 ΔH soln. Molar heat of solution molar enthalpy of solution has the units 2 J mol-1 or kJ mol-1.
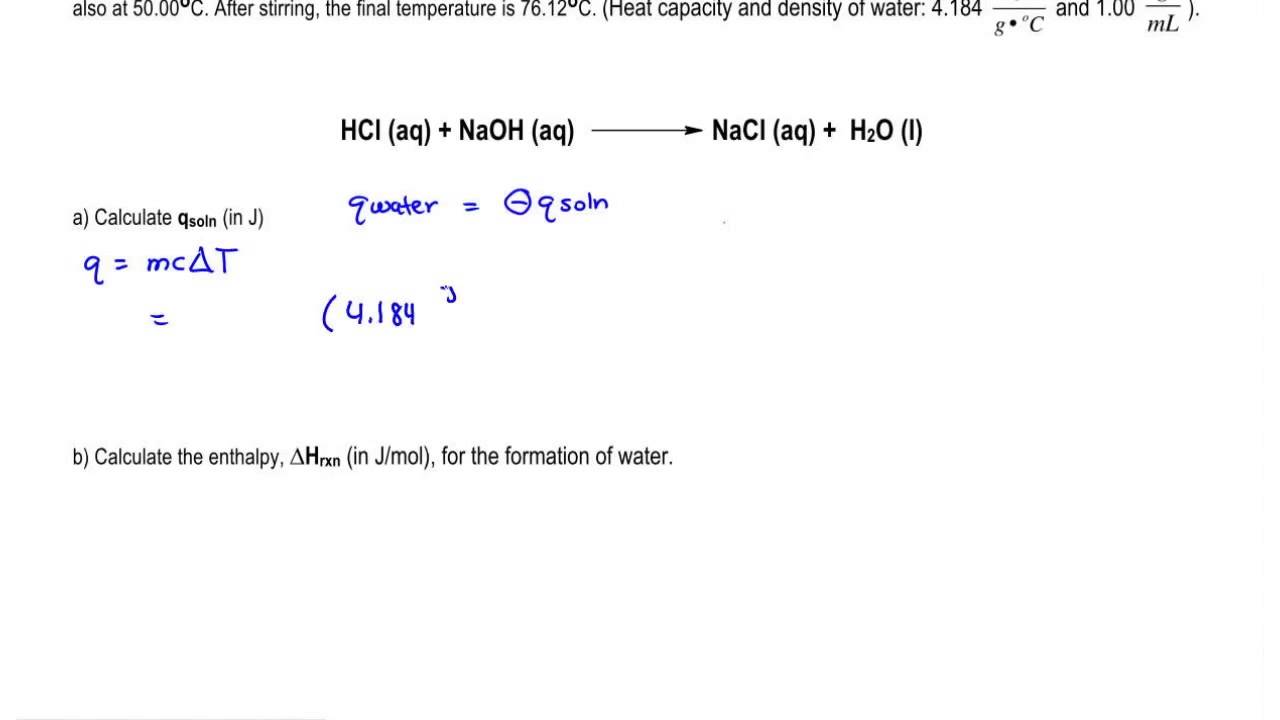
HClaq NaOHaq – NaClaq H 2 Ol Energy. Thermochemistry determine the heat exchanged at constant pressure q m c T. Calculating the limiting reactant the change in enthalpy of the reaction H rxn can be determined since the reaction was conducted under conditions of constant pressure H rxn q rxn moles of limiting reactant.
This demonstration illustrates how the. Special care is required to prepare a solution of sodium hydroxide or NaOH in water because considerable heat is liberated by the exothermic reaction. The solution may splatter or boil.
Here is how to make a sodium hydroxide solution safely along with recipes for several common concentrations of NaOH solution. The molecular weight of NaOH is 40. That means you need to dissolve 40 g of NaOH in water to obtain a 1 liter of 1M or 1N NaOH solution.
To prepare a 10M NaOH solution you need to dissolve 10 times more NaOH ie 400 g of NaOH for 1 L solution. Here we will prepare 100 ml of 10M NaOH solution. Therefore we need to take 40 g of NaOH.
Enthalpy of Solution Heat of Solution Example. In an experiment 12 g of sodium hydroxide pellets NaOH s were dissolved in 100 mL of water at 25C. The temperature of the water rose to 275C.
Calculate the enthalpy change heat of solution for the reaction in kJ mol-1 of solute. NaOH is insoluble in ether and other non-polar solvents. Similar to the hydration of sulfuric acid dissolution of solid sodium hydroxide in water is a highly exothermic reaction where a large amount of heat is liberated posing a threat to safety through the possibility of splashing.
The resulting solution is usually colorless and odorless. HEAT OF SOLUTION DATA FOR AQUEOUS SOLUTIONS Some heats of solutions and heats of hydration for dilute solutions in pure water at 15 ºC. Solute Products Heat of solution EXOTHERMIC CH.
2 l methanoic acid H aqCHO. 2-aq 086 kJmol C. 2 l acetic acid H aqC.
2-aq 15 kJmol CH. It makes a solution of sodium hydroxide also called caustic soda or lye in dissolving heat is produced - a lot of heat. Always add sodium hydroxide to water NaOH H2O Na and OH- ions.
The reaction will be Exothermic where heat will be released. The heat evolved as a result of mixing solid sodium hydroxide with water is due to the. A violent explosion resulted when a quantity of pentol was accidentally brought in contact with a caustic cleaning solution chemically similar to aqueous sodium hydroxide MCA Case History 363 1964.
Aqueous solutions of reducing sugars other than sucrose when heated above 84C evolve toxic levels of carbon monoxide in the presence of alkalis or alkaline salts such as sodium phosphate. The solution including the reactants and the products and the calorimeter itself do not undergo a physical or chemical change so we need to use the expression for specific heat capacity to relate their change in temperature to the amount of heat q cal that they have exchanged Eqn. 3 m is the mass mass of the reactants mass of water mass of calorimeter C is the.
Here we have 15 ml of a solution that is 06N this means we have 15X06 9 milliequivalents mEq of acid. Hence we will need 9 mEq of a base for a complete neutralization and we must get them from a 01N NAOH solution. Since 1 ml of.
The idea here is that you can use the heat absorbed by the solution to find the heat given off by the dissolution of the salt. More specifically you can assume that. DeltaH_diss -q_solution The minus sign is used here because heat lost carries a negative sign.
To find the heat absorbed by the solution you can use the equation. Such physical processes are classified into two types. Heat of solution and Heat of dilution.
The heat of solution or enthalpy of solution is defined as the heat generated or absorbed when a certain amount of the solute dissolves in a certain amount of solvent. It is noted that certain heat effects take place when a solution of a given concentration is. Measure the heat capacity of a Styrofoam cup calorimeter using the heat of neutralization of a strong acid with a strong base Graph your temperature vs time data to find temperature change when solutions are mixed PreBLaboratoryRequirements.
Read chapter 6 in Silberberg Pre-lab questions if required by your instructor Laboratory notebookprepared before lab if required. The chemical solution of 50 NaOH is denser than water with a density around 152gcm 3 at 68F. When dissolving solid NaOH in water or a strong acid the ionic dissociation process can be violently exothermic rapidly releasing significant heat energy.
Upon ionic dissociation free hydroxide ions OH. A strong base is a base that is completely dissociated in an aqueous solution. These compounds ionize in water to yield one or more hydroxide ion OH - per molecule of base.
In contrast a weak base only partially dissociates into its ions in water. Sodium hydroxide NaOH - Sodium hydroxide is an ionic compound. The molecular weight of sodium hydroxide is 40 gmol.
It is a white translucent crystalline solid and used in the manufacturing of detergents and soaps. To learn about the structure Properties Preparation Uses Health Hazards and FAQs of Sodium hydroxide NaOH. Visit BYJUS for more information.
HNO 3aq NaOHaq NaNO 3 aq H 2 Ol ΔH -573 kJ When 250 cm 3 of 10 mol dm-3 nitric acid is added to 200 cm 3 of 20 mol dm-3 sodium hydroxide solution what is the change in temperature. Specific heat capacity of solution. 42 J g-1 C-1.
1 g cm-3 Solution. Determine heat of neutralization of strong acid and strong. This reaction is an exothermic reaction heat is given to outside.
Ethanoic acid and NaOH. Sodium ethanoate and water are given as products by the reaction of ethanoic acid and aqueous NaOH. Sodium ethanoate is a salt and soluble in water.
PH of the solution is changed during the reaction. Titration of adding sodium hydroxide to ethanoic acid. Aqueous ethanoic acid shows a pH value below.
Also it is assumed that the specific heat capacity of the solution sp_heat is approximately equal to that of water sp_heat water 4184 JgC. The moles of water produced in the neutralization are calculated using the volume and concentration molarity of the acid and base solutions. Lastly the enthalpy of neutralization per mole of water produced is calculated ΔH in kJmol.
Heat this solution to 58 to 60 degrees Cdo not allow the solution to get to hot. Add 1N NaOH drop by dropuntil the solution turns clear. Add the phosphate buffer stock and allow to cool and then add the glutaraldehyde.
Ph the solution to 74 and FILTER before use. 1 Paraformaldehyde 2 Glutaraldehyde Hrp Fix 02M Stock Phosphate Buffer pH 74 500ml Paraformaldehyde 10g Glutaraldehyde. Production of high quality steam.
External treatment is the reduction or removal of impurities from water outside the boiler. In general external treatment is used when the amount of one or more of the feed water impurities is too high to be tolerated by the boiler system in question. There are many types of external treatment softening.
4 is added to 50 mL of 10 M NaOH at 25oC in a calorimeter the temperature of the aqueous solution increases to 339 oC. Assuming that the specific heat of the solution is 418 JgC that its density is 100 mL and that the calorimeter itself absorbs a negligible amount of heat calculate the amount of heat absorbed for the reaction. NaOHaq HClaq rarr H_2Ol NaClaq Why should I write this first.
Because it establishes the stoichiometry. The 11 molar equivalence. One equiv of sodium hydroxide reacts with one equiv hydrochloric acid.
Thus if I know the quantity of acid I also know the quantity of base. There will be reactions where a different stoichiometry. Determining the Heat of Reaction in Aqueous Solution.
In this activity students perform an experiment to determine the heat of a reaction. Get Info Go. Use the virtual lab to determine how much milk to add to hot coffee to reach the desired temperature.
Get Info Go. Measuring the heat capacity of an engine coolant. As an analytical chemist at a company developing new.