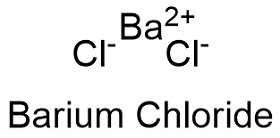
Except where otherwise noted data are given for materials in their standard state at 25 C 77 F 100 kPa. Therefore the toxic effects of ingested barium hydroxide may be considered to be similar to those observed with barium chloride.
The chemical formula of a compound is a symbolic representation of its chemical composition.
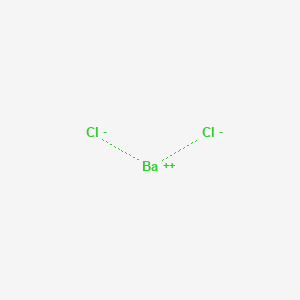
Formula for barium chloride. Barium chloride is an inorganic compound with the formula Ba Cl 2. It is one of the most common water-soluble salts of barium. Like most other barium salts it is white toxic and imparts a yellow-green coloration to a flame.
It is also hygroscopic converting first to the dihydrate BaCl 2 H 2 O 2. It has limited use in the laboratory and industry. Barium stimulates striated cardiac and smooth muscle regardless of innervation.
It is antagonistic to all muscle depressants no matter whether they act primarily on nerve or muscle. Initial stimulation of contraction leads to vasoconstriction through direct action on arterial muscle peristalsis through action on smooth muscle tremors and cramps through action on the skeletal muscles and. The formula unit for the formation of potassium chloride and barium sulfate is one mole.
One unit of potassium sulfate and barium chloride are required for the reaction. The chemical formula of a compound is a symbolic representation of its chemical composition. Chemical formulae provide insight into the elements that constitute the molecules of a compound and also the ratio in which the atoms of these elements combine to form such molecules.
Ionic Compound Naming and Formula Writing List 1. Copy this to my account. E-mail to a friend.
Using this program will help you to learn how to write ionic compound names and formulas for Chemistry A. The reaction between Ammonium chloride and Barium hydroxide is an endothermic reaction. BaOH 2 2NH 4 Cl BaCl 2 2NH 3 2H 2 O.
Ammonium Chloride is slightly acidic and Barium Hydroxide very basic so will get an acid-base reaction. This reaction is used to recover ammonia in the preparation of sodium carbonate from NaCl. The ionic compound without the waters of hydration is named first by using the rules for naming ionic compounds eg BaOH 2 8H 2 O barium hydroxide.
Greek prefixes are attached to the word hydrate to indicate the number of water molecules per formula unit for the compound eg BaOH 2 8H 2 O. 8 water molecules octa hydrate. Barium chloride Radium chloride.
Except where otherwise noted data are given for materials in their standard state at 25 C 77 F 100 kPa. What is Infobox references. Magnesium chloride is the name for the chemical compound with the formula MgCl 2 and its various hydrates MgCl 2 H 2 O x.
Anhydrous MgCl 2 contains 255 elemental magnesium by mass. These salts are typical ionic. Ionic Compounds Naming and Formula Writing.
Copy this to my account. E-mail to a friend. This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central.
Ionic Compound Formula Writing Worksheet Write chemical formulas for the compounds in each box. The names are found by finding the intersection between the cations and anions. The first box is the intersection between the zinc cation and the chloride anion so you should write ZnCl 2 as shown.
Zinc iron II iron III gallium silver lead IV chloride ZnCl 2. Nitrate group and barium combines with two nitrates parentheses are placed around the nitrate and the subscript 2 indicates two nitrate groups. Changing the subscripts in the nitrate to N 2O6 would chang e the meaning of the formula and is incorrect.
Counting atoms barium nitrate consists of one barium atom two nitrogen atoms and six oxygen atoms. In the compound zinc phosphate. The calculator can be used to calculate the chemical formula of a range of 1.
The chemical formula of ionic compounds can be quickly calculated using the chemical formula calculator. An ionic compound is composed of a metal and a non. Binary Ionic Formula Practice Name_____ 1 sodium chloride Na1 Cl-1 NaCl 2 lithium bromide Li1 Br-1 LiBr 3 magnesium flouride Mg2 F-1 MgF2 4 potassium oxide K1 O-2 K2O 5 calcium sulfide Ca2 S-2 CaS 6 aluminum iodide Al3 I-1 AlI3 7 barium bromide Ba2 Br-1 BaBr2 8 aluminum sulfide Al3 S-2 Al2S 3 9 calcium phosphide P-3 Ca2 P-3.
The Mass percent formula is expressed as solving for the molar mass also for the mass of every element in 1 mole of the compound. Youll determine the mass percentage of every element with these masses. For a solution the mass percent is expressed because the grams of solute divided by the grams of solution then multiplied by 100 to urge a percentage.
BaOH2 barium hydroxide 16. PbCl2 lead II chloride 7. Na2S sodium sulfide 17.
KNO3 potassium nitrate 8. Iron II chloride 18. MgOH2 magnesium hydroxide 9.
Na2CrO4 sodium chromate 19. LiCIO3 lithium chlorate 10. NH42SO4 ammonium sulfate 20.
NiS nickel II sulfide Write the chemical formula for each of the following compounds. Answer 1 of 5. When you get questions like this it helps a lot if you can see the general sorts of patterns.
I will give the lowest level of explanation and then follow up with a high level explanation. In this case both of the reactants are salts. They are each made of a ca.
After ingestion barium hydroxide reacts with the HCl in the stomach to form water and the very soluble barium chloride. Therefore the toxic effects of ingested barium hydroxide may be considered to be similar to those observed with barium chloride. Due to the neutralizing effect of the HCl in the stomach no significant effects are expected from the alkalinity of BaOH2.
What is the correct name for the ionic compound NaClO 3. The formula unit for the ionic compound barium carbonate consists of which of the following. 2 Ba ions and 1 CO 3 2-ion.
1 Ba 2 ion and 2 CO 3-ions. 1 Ba 2 ion and 1 CO 3 2-ion. 1 Ba 2 ion and 2 HCO 3-ions.
1 Ba ion and 1 CO 3-ion. The formula unit for the. A 500 g sample of hydrated barium chloride BaCl 2 nH 2 O is heated to drive off the water.
After heating 426 g of anhydrous barium chloride BaCl 2 remains. What is the value of n in the hydrates formula. 1 Calculate moles of anhydrous barium chloride.
426 g 208236 gmol 0020458 mol. 2 Calculate moles of. Write balanced formula unit total ionic and net ionic equations for the following reactions.
Assume all reactions occur in water or in contact with water. Silver nitrate and Rubidium chloride. AgNO 3 aq RbClaq – AgCls RbNO 3 aq Total Ionic Equation.
Ag aq NO 3-aq Rb aq Cl-aq- AgCl s Rb aq NO 3-aq Net Ionic Equation. Ag aq. Ionic Compound Formula K sp.
Aluminum hydroxide AlOH 3 1810 5. Aluminum phosphate AlPO 4 6310 19. Barium carbonate BaCO 3 5110 9.
Barium chromate BaCrO 4 1210 10. Barium fluoride BaF 2 1010 6. Barium hydroxide BaOH 2 510 3.
Barium sulfate BaSO 4 1110 10. Barium sulfite BaSO 3 810 7. Barium thiosulfate BaS 2 O 3 1610 6.
The molecular formula for glucose is C₆H₁₂O₆ which tells us the exact number of constituent atoms carbon hydrogen and oxygen written as C H and O respectively in one glucose molecule. The empirical formula of glucose is CH₂O which represents the whole number ratio of the constituent atoms viz C H and O. Structural formula of glucose will indicate how each carbon hydrogen and.
Thus the Cl-ion is called chloride the S 2-ion is called sulfide. Al 3 Aluminum. Ag Silver.
Binary Ionic Compounds Type II The cation of a transition metal is always named first like any cation and the anion second. A monatomic meaning one-atom cation takes its name from the name of the element. Im glad you found your way here for chemistry help because youre in the right spot to be enlightened.
By the way if its not actually the holidays and I havent updated this recently sorry about that. Here are the rules for using this free website. I promise that I wont take your.
Empirical Formula Hill Notation. C 25 H 42 N 7 O 17 P 3 S xLi CAS No. 83762 free acid basis Compare Product No.
Ac-CoA Synthase Inhibitor - CAS 508186-14-9 - Calbiochem. Ac-CoA Synthase Inhibitor - CAS 508186-14-9 - Calbiochem.