Proteins remain in aqueous solution because of interactions between the hydrophilic water-loving amino acids and the surrounding water molecules the solvent. The derivatives thus identified were characterized by GC high resolution MS deuterium exchange for active hydrogen in the chemical ionization source of the mass spectrometer and GCMS following on-column base catalyzed.

When haemoglobin was added to the electrodes the current intensity of the ferrocene in the sensor.
Ferrocene and water. Ferrocene is an organometallic compound with the formula FeC 5 H 5 2. The molecule is a complex consisting of two cyclopentadienyl rings bound to a central iron atom. It is an orange solid with a camphor-like odor that sublimes above room temperature and is soluble in most organic solvents.
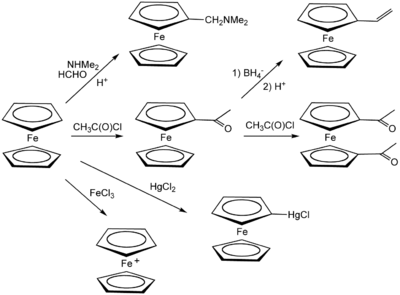
It is remarkable for its stability. It is unaffected by air water strong bases and can be heated. Ferrocene does not undergo addition reactions typical of cyclopentadiene but readily undergoes electrophilic aromatic substitution.
Depending upon the catalyst and the reaction conditions the major acetylation product is either the monosubstituted product 2 or the disubstituted product 3. For a particular set of reaction conditions the student will determine whether the major product is the. Filter the precipitate and wash with water.
Collect the crude orange ferrocene and dry in the air. Purify by sublimation to obtain orange crystalline material and record your yield as shown in Figure 3. Experimental setup for ferrocene synthesis.
Purification of crude ferrocene via. And wash with water. Collect the crude orange ferrocene and dry in the air.
Purify by sublimation to obtain orange crystalline material and record your yield see Figure 32 Preparation of ferrocene There are two parts to this experiment. First prepare freshly distilled cyclopentadiene C5H6 and second use it immediately to synthesise ferrocene. Both parts should be done in the fumehood.
Ferrocene derivatives have been used in a wide number of material science applicationsso diverse are they that it is difficult to compartmentalize these into a coherent text. Thus this is a general overview of some specific applications. At the outset it is worth noting that in many cases ferrocene acts as a reagent in synthesis specific examples would be in the preparation of nanotubes.
The water can be removed by heating for a few hours at 300C. A molecular sieve of pore size 05. Molecular sieves with different structure and concentration of acid sites were investigated in liquid phase acylation of ferrocene with derivatives of acetic acid.
It was shown that conversion of ferrocene is strongly related to the size and dimensionality of zeolite channel systems. Aptamers that specifically bind HbA1c and Hb were modified with a sulfhydryl and ferrocene group at the 3 and 5-end respectively. The modified aptamers were coated through sulfhydryl-gold self-assembly onto screen printed electrodes producing aptasensors with built in electroactivity.
When haemoglobin was added to the electrodes the current intensity of the ferrocene in the sensor. Determination of Molar Absorption Coefficient. Select a blank cuvette and place it in the spectrophotometer.
Click on 0 ABS 100T button the instrument now reads 000000 A. Choose a solution with known concentration and measure the absorbance between the wavelengths 350 nm to 700 nm. Record the wavelength at the.
Electrodes were then treated under ultrasound in 100 ethanol and ultrapure water for 3 min. Subsequently the electrodes were rinsed under ultrasound sequentially in 05 mmolL H 2 SO 4 01 mmolL KCl and ultrapure water followed by drying with N 2 prior to use. HbA1c or Hb aptamer was diluted to a final concentration of 5 μmolL with TM.
A QRE with ferrocene or another internal standard such as cobaltocene or decamethylferrocene referenced back to ferrocene is ideal for nonaqueous work. Since the early 1960s ferrocene has been gaining acceptance as the standard reference for nonaqueous work for a number of reasons and in 1984 IUPAC recommended ferrocene IIIII as a standard redox couple. The ZrIV-based MOF solid boasts multiple key functions.
1 a dense array of alkyne units over the backbone and the side arms which are primed for extensive graphitization. 2 the open branched structure helps maintain porosity for absorbing nitrogen dopants. And 3 ferrocene units on the side arms as atomically dispersed precursor catalyst for targeting micropores and for effective iron.
TLC NMR and IR spectroscopy were used throughout the process to identify ferrocene and acetylferrocene in addition to evaluating the levels of purity. The objective of our experiments was to prepare acetylferrocene from ferrocene. The overall reaction was carried out using 61 equivalents of liquid acetic anhydride to 18 equivalents of phosphoric acid and concluded with an aqueous.
Synthesis of molecular wires 1a 1b. And 2 1 H and 13 C spectra potential energy surface as a function of ferrocene dihedral angle in compounds 1a and 1b plateau displacement distribution analysis for 1a 2D conductance histograms for each molecular wire 1D conductance histograms for each molecular wire showing G 0 peaks conductance traces and 1D conductance histogram for blank. Welcome to the Molecule of the Month page.
This is one of the longest running chemistry webpages on the internet. Each month since January 1996 a new molecule has been added to the list on this page which makes this one of the longest running Chemical websites on the internet. The links will take you to a page at one of the Web sites at a University Chemistry Department or commercial site in.
Most ionic liquids cannot tolerate water and hence an SCE or AgAgCl reference electrode cannot be used. When working with ionic liquids it is typically advisable to use a Ag wire when possible. In cases such as this potentials are simply reported against the AgAg couple although it is best to dissolve a known compound such as ferrocene in the ionic liquid afterward and report potential.
A ferrocene functionalized Schiff base containing CuII complex. Synthesis characterization and parts-per-million level catalysis for azide alkyne cycloaddition Direct synthesis of ortho-methylthio allyl and vinyl ethers via three component reaction of aryne activated alkene and DMSO. Determining the Free Chlorine Content of Swimming Pool Water.
Go Direct Colorimeter. Determining the Quantity of Iron in a Vitamin Tablet. Go Direct Colorimeter.
Determining the Phosphoric Acid Content in Soft Drinks. Go Direct pH Sensor. Go Direct pH Sensor.
Click on theExperiment Title link to the lab that you wish to. The point group of the water molecule We start at the top of the flow-chart and can see that the water molecule is not linear and is not tetrahedral Td octahedral Oh or icosahedral Ih so we proceed down the chart 15. C2 Yes there is a principal Cn axis so we proceed down the chart but in answer to the next question there are no further C2 axes at right angles to the principal.
Acetylation of ferrocene essay writing. Cheap definition essay writer service for school. Cheap best essay editing service online.
Apa structure essay example. Best scholarship essay editor for hire for phd. Argumentative essay the yellow wallpaper.
Cheap personal essay editing for hire gb. Beowulf book and movie comparison essay. As u sow so shall reap essay.
Best essays for grad school for. Water electrolysis excels the traditional petrochemical techniques in terms of. Recent advances in applications of voltammetric sensors modified with ferrocene and its.
Ground water extracts were analyzed by GCMS to determine if derivatives of dicyclopentadiene were present in addition to dicyclopentadiene and other chemical wastes. The derivatives thus identified were characterized by GC high resolution MS deuterium exchange for active hydrogen in the chemical ionization source of the mass spectrometer and GCMS following on-column base catalyzed. The most remarkable properties of ferrocene and its structure and chemistry are emphasized from historical aspects to recent trends in an overview justifying why it is such an exceptional tool in asymmetric catalysis electron-transfer processes sensing molecular engineering opto-electronics materials science and medicine.
The ALD process was carried out on a PtSiO 2 catalyst Supplementary Fig. 1 by alternately exposing the sample to ferrocene FeCp 2 and. Proteins remain in aqueous solution because of interactions between the hydrophilic water-loving amino acids and the surrounding water molecules the solvent.
If the ionic strength of the solvent is increased by adding an agent such as ammonium sulfate some of the water molecules will interact with the salt ions thereby decreasing the number of water molecules available to interact with.