Whenaddedto an infusion bag containing 09 sodium chloride injection USP at concentrations ranging from 2 mg to 4 mg of iron per mL Injectafersolution is. Ferric Carboxymaltose INJECTAFER Generic.
The adjective ferric or the prefix ferri- is often used to specify such compounds as in ferric chloride for ironIII chloride FeCl 3.
Ferric chloride reaction with iron. IronIII chloride is the inorganic compound with the formula Fe Cl 3. Also called ferric chloride it is a common compound of iron in the 3 oxidation state. The anhydrous compound is a crystalline solid with a melting point of 3076 C.
The color depends on the viewing angle. By reflected light the crystals appear dark green but by transmitted light they appear purple-red. Ferric chloride is a common compound of iron and chlorine in which iron possesses 3 oxidation state.
Its IUPAC name is IronIII chloride or Iron trichloride. Apart from ferric chloride it has several common names such as molysite and flores martis. It possesses various colours such as in its anhydrous form it appears black-green or purple while in its hydrous form it appears yellow solid.
Ferric chloride is an orange to brown-black solid. It is slightly soluble in waterIt is noncombustible. When wet it is corrosive to aluminum and most metals.
Pick up and remove spilled solid before adding waterIt is used to treat sewage industrial waste to purify water as an etching agent for engraving circuit boards and in the manufacture of other chemicals. The adjective ferric or the prefix ferri- is often used to specify such compounds as in ferric chloride for ironIII chloride FeCl 3. The adjective ferrous is used instead for ironII salts containing the cation Fe 2.
The word ferric is derived from the Latin word ferrum for iron. IronIII metal centres also occur in coordination complexes such as in the anion ferrioxalate Fe. Preparation of Ferric Chloride.
Anhydrous iron III chloride can be prepared by reacting metallic iron with dichloride. The chemical equation for this reaction is provided below. 2Fe 3Cl 2 2FeCl 3.
Preparation of Ferric Chloride Solution. By dissolving iron ore in HCl hydrochloric acid Fe 3 O 4 8HCl FeCl 2 2FeCl 3 4H 2 O. By oxidizing iron II chloride with chlorine.
Iron Ferric Carboxymaltose Infusion. Confirmed previous reaction to intravenous iron to be assessed by Medical Practitioner Anaemia not attributed to iron deficiency eg. Other causes of microcytic anaemia.
Evidence of iron overload or disturbances in utilisation of iron. Pregnancy in the first trimester. Dosage The adequate cumulative dose of Ferinject must be calculated for each.
Each 2 mL vial contains 100 mg of iron as ferric carboxymaltose. Each 10 mL vial contains 500 mg of iron as ferric carboxymaltose. Each 20 mL vial contains 1000 mg of iron as ferric carboxymaltose.
Excipients with known effect. One mL of solution contains up to 55 mg 024 mmol sodium see section 44. For the full list of excipients see section 61.
FERRIC NITRATE is an oxidizing agent. Mixtures with alkyl esters may explode owing to the formation of alkyl nitrates. Mixtures with phosphorus tinII chloride or other reducing agents may react explosively Bretherick 1979 p.
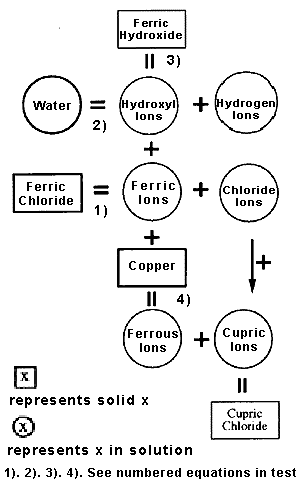
Aqueous ferric chloride and aqueous sodium hydroxide reaction. Ferric chloride IronIII chloride is a soluble inorganic compound in water and form a yellow-brown solution. Sodium hydroxide is a strong alkaline colorless solution and readily gives hydroxyl ions OH-.
What are the products when aqueous FeCl 3 reacts with aqueous NaOH. When we add aqueous NaOH solution to aqueous FeCl 3. Each 2 mL vial contains 100 mg of iron as ferric carboxymaltose.
Each 10 mL vial contains 500 mg of iron as ferric carboxymaltose. Each 20 mL vial contains 1000 mg of iron as ferric carboxymaltose. Excipients with known effect.
Sodium hydroxide for pH adjustment. For the full list of excipients see Section 61 List of Excipients. 250 mL of sterile 09 sodium chloride injection USPsuch that the concentration of the infusion is not less than 2 mg of iron per mL and administer over at least 15 minutes.
Whenaddedto an infusion bag containing 09 sodium chloride injection USP at concentrations ranging from 2 mg to 4 mg of iron per mL Injectafersolution is. Silver chloride decomposes in presence of sunlight to form silver metal and chlorine gas. Iron sulphate which is of green colour is decomposed to form a brown-coloured ferric oxide and gases like sulphur dioxide and sulphur trioxide gases are released.
Lead nitrate on thermal decomposition gives lead monoxide which is yellow. With this brown gas nitrogen dioxide and oxygen gas are released. A Ferric chloride test Dissolve a drop or a few small crystals of the compound in 1 mL of 95 ethanol rectified spirit and add 1 mL of M hydrochloric acid.
Note the colour produced when 1 drop of 5 iron III chloride is added to the solution. If a pronounced violet blue red or orange colour is produced the hydroxamic acid test. The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way.
The primary aim of the cards is to promote the safe use of chemicals in the workplace. The main target users are workers and those responsible for occupational safety and health. The ICSC project is a common undertaking between the World Health Organization WHO and.
It forms when iron and oxygen react in water or in moisture in the air. The reaction of iron and chloride underwater is also referred to as rust. Certain factors speed up the rusting process such as salt in the water.
Didnt Read Rusting is a common form of corrosion which occurs when metal atoms react with their environment. Salt water does not make a metal rust but it. Ferric Ammonium Citrate.
Final pH at 25C. Principle of Triple Sugar Iron TSI Agar. The TSI agar is a special medium with multiple sugars constituting a pH-sensitive dye phenol red 1 lactose 1 sucrose 01 glucose as well as sodium thiosulfate and ferrous sulfate or ferrous ammonium sulfate.
All of these. Ferric Carboxymaltose INJECTAFER Generic. Ferumoxytol FERAHEME Infusion Generic.
Filgrastim-Sndz ZARXIO for Hepatitis C Generic. Filgrastim-Sndz ZARXIO for Stem Cell Mobilization Generic. Golimumab Simponi Aria Generic.
Last updated on July 1st 2021. Kliglers Iron Agar KIA is used for the detection of carbohydrate fermentation. Reactions of KIA helps to includeexclude particular bacterial isolate in the family Enterobacteriaceae.
If an organism cannot ferment glucose then in KIA alkaline slant-alkaline-butt no change reaction is observed this reaction alone is sufficient to exclude an isolate. The Fenton reaction has several advantages compared to other AOPs. First of all iron and hydrogen peroxide H 2 O 2 are abundant and safe from an environmental point of view if optimally dosedFurthermore the process follows relatively simple operating principles and the absence of mass transfer limitations Mirzaei et al 2017In the case of the photo-Fenton reaction the exposure to.
Chloride Chloride concentrations above 100 mgL cause low results. The test can be used at high chloride concentrations seawater if a calibration is made with standards that have the same chloride concentration as the samples refer to Seawater calibration on page 5. Ferric iron Interferes at all levels Nitrite Interferes at all levels.
Ferric Chloride and Aluminum Chloride Catalyzed Chlorination of Benzene Alkylbenzenes and Halobenzenes George A. Kuhn and Barbara A. Hardie Journal of the American Chemical Society 1964 86 6 1055-1060 DOI.
1963 43 15 DOI. 1015227orgsyn0430015 Since anthracene is significantly. When ferric hydroxide is heated it decomposes into ferric oxide and water 2FeOH 3 s underrightarrow triangle Fe 2 O 3 s 3H 2 Ol Thermal Decomposition.
The decomposition of a substance on heating is known as Thermal Decomposition. 2PbNO 3 2 s underrightarrow heat 2PbOs 4NO 2 g O 2 g Electrolytic Decomposition. Reactions in which compounds.
Ferric chloride or sulphate and ferrous sulphate also know as copperas are all widely used for phosphorous removal although the actual reactions are not fully understood. The basic reaction is. Fe 3 H n PO 4 3-n FePO 4 nH Ferric ions combine to form ferric phosphate.
Potassium chloride vs salt. Plus brine tank maintenance tips. But the coarse mix used on roads and highways contains high levels of chemicals such as sodium ferrocyanide and ferric ferrocyanide that prevent caking during storage.
Purchasing bags of high-quality evaporated salt pellets is your best bet. Youll spend less time cleaning impurities out of your brine tank. Feraheme when added to intravenous infusion bags containing either 09 Sodium Chloride Injection USP normal saline or 5 Dextrose Injection USP at concentrations of 2-8 mg elemental iron per mL should be used immediately but may be stored at controlled room temperature 25C 2C for up to 4 hours or refrigerated 2-8 C for up to 48 hours.
The dosage is expressed in terms of.