
Carbon dioxide CO 2 is one of the few examples. Monosodium glutamate has chemical formula as.
Water is the most abundant molecule in cells accounting for 70 or more of total cell mass.
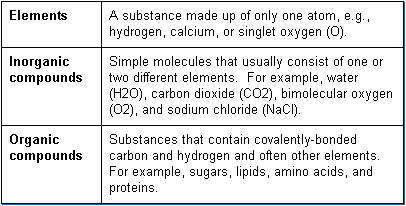
Examples of inorganic compounds human body. Inorganic compounds are those which are not organic that is they are without a carbon-hydrogen bond. Discover the characteristics applications and examples of these compounds. In contrast only a handful of inorganic compounds contain carbon atoms.
Carbon dioxide CO 2 is one of the few examples. An organic compound is a substance that contains both carbon and hydrogen. Organic compounds are synthesized via covalent bonds within living organisms including the human body.
Recall that carbon and hydrogen are the. In contrast only a handful of inorganic compounds contain carbon atoms. Carbon dioxide CO 2 is one of the few examples.
An organic compound then is a substance that contains both carbon and hydrogen. Organic compounds are synthesized via covalent bonds within living organisms including the human body. Recall that carbon and hydrogen are.
Examples of Inorganic Compounds. Inorganics include salts metals substances made from single elements and any other compounds that dont contain carbon bonded to hydrogen. Some inorganic molecules do in fact contain carbon.
Table salt or sodium chloride NaCl. Carbon dioxide CO 2. Diamond pure carbon silver.
Organic Compounds Without C-H Bonds. The element carbon forms the basic unit of organic inorganic and organometallic compounds. Right at the moment when you go to sleep till you wake up infinite chemical processes are taking place in each cell of your body.
Even processes occur when you wake up all your daily activities like drinking water taking a shower cooking your food cleaning your car laughing or crying are guided. Enzymes in the human body serve a variety of different functions. For example different categories of enzymes include digestive enzymes metabolic enzymes liver enzymes nucleases and receptor enzymesExamining these five categories of enzymes in detail will make the many important roles of enzymes in the human body more clear.
Carbon compounds are chemical substances that contain carbon atoms bonded to any other element. There are more carbon compounds than for any other element except hydrogenThe majority of these molecules are organic carbon compounds eg benzene sucrose although a large number of inorganic carbon compounds also exist eg carbon dioxideOne important characteristic. The body obtains carbohydrates from plant-based foods.
Grains fruits and legumes and other vegetables provide most of the carbohydrate in the human diet although lactose is found in dairy products. Although most body cells can break down other organic compounds for fuel all body cells can use glucose. Moreover nerve cells neurons in the.
Salt plays a crucial role in maintaining human health. It is the main source of sodium and chloride ions in the human diet. Sodium is essential for nerve and muscle function and is involved in the regulation of fluids in the body.
Whereas chloride ions serve as important electrolytes by regulating blood pH and pressure. Lead also is very resistant to weather conditions. Lead and lead compounds are toxic and can present a severe hazard to those who are overexposed to them.
Whether ingested or inhaled lead is readily absorbed and distributed throughout the body. Until 1978 lead compounds were an important component of many paints. Lead was added to paint to.
Mercury poisoning is a type of metal poisoning due to exposure to mercury. Symptoms depend upon the type dose method and duration of exposure. They may include muscle weakness poor coordination numbness in the hands and feet skin rashes anxiety memory problems trouble speaking trouble hearing or trouble seeing.
High-level exposure to methylmercury is known as Minamata disease. Cells are composed of water inorganic ions and carbon-containing organic molecules. Water is the most abundant molecule in cells accounting for 70 or more of total cell mass.
Consequently the interactions between water and the other constituents of cells are of central importance in biological chemistry. The critical property of water in this respect is that it is a polar molecule in. It is categorized as a non-essential amino acid means it can be synthesized by human body.
Monosodium glutamate has chemical formula as. C 5 H 8 NO 4 Na. Sodium hydroxide is an inorganic compound composed from sodium Na and hydroxde OH.
It is colorless solid and categorized as salt. Define functional groups and give examples. Animal tissues plant tissues bacteria and fungi contain organic molecules.
Horns and nails fallen leaves eggs fruits and vegetables contain organic compounds. Wood milk paper petroleum and gasoline contain organic compounds. In summary all living matter parts or products of living matter and remains of living matter contain organic.
Most organic compounds contain carbon and hydrogen but they may also include any number of other elements eg nitrogen oxygen halogens phosphorus silicon sulfur. Originally limited to the study of compounds produced by living organisms organic chemistry has been broadened to include human-made substances eg plastics. The average 70 kg 150 lb adult human body contains approximately 7 10 27 atoms and contains at least detectable traces of 60 chemical elements.
About 29 of these elements are thought to play an active positive role in life and health in humans. The relative amounts of each element vary by individual mainly due to differences in the proportion of fat muscle and bone in their body. Some of these compounds seem to have people known for ages because of their wide range of activity on human organisms and also other animals.
For thousand years extracts from plants containing alkaloids had medicinal use as drugs and they owe their powerful effects thanks to the presence of alkaloids. Morphine was the first alkaloid which was isolated about 1804 from opium. Diethyl ether BP 345 degree centigrade b Non-Volatile type.
Most of the solvents come under this category. They have high boiling points and hence are stable at room temperature. They get evaporated when heated to higher temperatures depending on their boiling points.
Water BP 100 degrees ethanol BP78 degrees. Examples include your stomach small intestine and airways in the lungs. Cells lining these body cavities secrete or send out glycoproteins.
The sugars mixed with water in your body create a. Organic Chemistry - In simple terms it is scientific approach to study of structure composition and diverse properties of organic compounds that contain carbon. From reactions to synthesis proper study of this area is essential for veterinarians dentists chemical engineers specialists that work with living organisms.
Real life examples include plastics foods fuels that are constantly. EPA has set standards for more than 80 contaminants that may occur in drinking water and pose a risk to human health. The contaminants fall into two groups according to the health effects that they cause.
Acute effects occur within hours or days of the time that a person consumes a contaminant. People can suffer acute health effects from almost any contaminant if they are exposed to.