
Therefore the following measures were agreed for feed following the finding of ETO sum of ethylene oxide and 2-chloroethanol expressed as ethylene oxide. Léthylène glycol ou glycol ou encore éthane-12-diol est le plus simple composé chimique de la famille des glycols.
Ethane-12-diol is an organic compound with the formula CH 2 OH 2.
Ethylene glycol properties. Ethylene glycol IUPAC name. Ethane-12-diol is an organic compound with the formula CH 2 OH 2. It is mainly used for two purposes as a raw material in the manufacture of polyester fibers and for antifreeze formulations.
It is an odorless colorless sweet-tasting toxic viscous liquid. Ethylene glycol is produced from ethylene ethene via the intermediate. Ethylene glycol dinitrate abbreviated EGDN and NGc also known as nitroglycol is a chemical compound a colorless oily explosive liquid obtained by nitrating ethylene glycolIt is similar to nitroglycerin in both manufacture and properties though it is more volatile and less viscousUnlike nitroglycerine the chemical has a perfect 0 oxygen balance meaning that its ideal exothermic.
Ethylene glycol is also commonly used in heating applications that temporarily may not be operated cold in surroundings with freezing conditions - such as cars and machines with water cooled engines. Ethylene Glycol is the most common antifreeze fluid for standard heating and cooling applications. Ethylene glycol should be avoided if there is a slightest chance of leakage to potable water or.
Ethylene Glycol C2H6O2 - Ethylene glycol is the first member of the series of alkane diols and is also known as glycol. Ethylene glycol is a colorless liquid with the chemical formula C2H6O2. The molar mass of ethylene glycol is 6207 gmol.
Mono ethylene glycol also known as MEG EG 12-ethanediol or 12-Dihydroxyethane is an organic compound with the formula C 2 H 6 O 2. It is a slightly viscous liquid with a clear colourless appearance and a sweet taste that emits virtually no odour. Its miscible with water alcohols and many other organic compounds and is primarily used in the industry for manufacturing polyester.
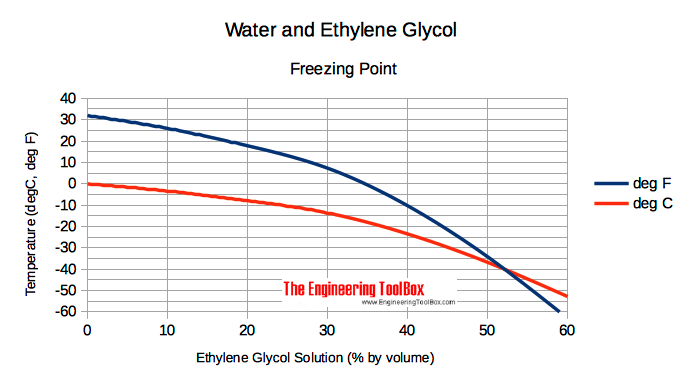
Ethylene Gas - Specific Heat - Specific heat of Ethylene Gas - C 2 H 4 - temperatures ranging 175 - 900 K. Ethylene Glycol Heat-Transfer Fluid Properties - Properties like freezing point viscosity specific gravity and specific heat of ethylene glycol based heat-transfer fluids or brines. Ethylene glycol as an antifreeze is based on its ability to lower the freezing point when mixed with water.
The physical properties of ethylene glycol-water mixtures are therefore extremely important. The end uses for ethylene glycol are numerous See Table 1. Ethylene Glycol The Versatile Performer.
Ethylene glycol possess far superior heat transfer properties whereas Propylene Glycol carries a very low toxicity. A glycol is an organic chemical compound belonging to the alcohol family. Within the glycol another terms for diol molecule it contains two hydroxyl groups attached to different carbon atoms.
Glycols belong in the alcohol group of chemicals. Polyethylene glycol PEG is a hydrophilic polymer. It can be easily synthesized by the anionic ring opening polymerization of ethylene oxide into a range molecular weights and variety of end groups.
When crosslinked into networks PEG can have high water content forming hydrogels. Hydrogel formation can be initiated by either crosslinking PEG by ionizing radiation or by covalent. Mono Ethylene Glycol 9 PHYSICAL AND CHEMICAL PROPERTIES APPEARANCE Liquid COLOUR Colourless ODOUR Odourless SOLUBILITY Miscible with water.
Miscible with Acetone Alcohol BOILING POINT C196 - 199 760 mm HgMELTING POINT C-12 RELATIVE DENSITY1115 20 c VAPOUR DENSITY air1214 VAPOUR PRESSURE0007 kPa 20 c pH-VALUE DILUTED SOLUTION. Propylene glycol also called 12-propanediol resembles ethylene glycol in its physical properties. Unlike ethylene glycol however propylene glycol is not toxic and is used extensively in foods cosmetics and oral hygiene products as a solvent preservative and moisture-retaining agent.
Propylene glycol is manufactured in large amounts from propylene oxide which is obtained from. A 6040 ethylene glycolwater mixture cooled to -40 C can chill an item at 20 C much more quickly and efficiently than pure water at 10 C. Although ethylene glycol has a lower heat capacity than water each kilogram of glycol is easier to heat than a kilogram of water the larger temperature difference allows a glycol mixture to carry heat away much more quickly than pure water.
Ethylene glycol is a clear colorless syrupy liquid. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment.
Since it is a liquid it can easily penetrate the soil and contaminate groundwater and nearby streams. Ethylene glycol is a synthetic liquid substance that absorbs water. It is odorless but has a sweet.
Ethylene glycol is more acutely toxic for humans than for laboratory animals by ingestion. The single oral lethal dose for humans has been estimated at 14 mlkg 156 gkg or about 100 ml 111 g for an adult. Not listed by ACGIH IARC NTP or CA Prop 65.
Ethylene oxide is a flammable gas with a somewhat sweet odor. It dissolves easily in waterEthylene oxide is a man-made chemical that is used primarily to make ethylene glycol a chemical used to make antifreeze and polyester. A small amount less than 1 is used to control insects in some stored agricultural products and a very small amount is used in hospitals to sterilize medical.
Mono-ethylene glycol - or MEG - is a vital ingredient for the production of polyester fibres and film polyethylene terephthalate. The humectant water attracting properties of MEG products also make them ideal for use in fibres treatment paper adhesives printing inks leather and cellophane. MEG is a colourless odourless liquid with a syrup-like consistency.
55 of MEG is used to make. Léthylène glycol ou glycol ou encore éthane-12-diol est le plus simple composé chimique de la famille des glycols. Sa formule semi-développée est HOCH 2 CH 2 OH et sa formule brute C 2 H 6 O 2 cest le plus simple des diols.
Léthylène glycol est fréquemment employé en tant quantigel dans le liquide de refroidissement des automobiles. À température ambiante cest un. We would like to show you a description here but the site wont allow us.
The drug-loading dual-targeted nanoparticles were prepared through emulsionsolvent evaporation technique. The Z-average particle size of d GluNPPTX was about 71 nm Fig. 2B without a significant difference compared with its unloaded counterpart Fig.
Such nanoparticles may accumulate more readily in tumor due to the enhanced permeability and retention EPR effect. It is a butyl ether of ethylene glycol and is miscible with water and common organic solvents. It has been produced industrially for over half a century and is used primarily as a solvent in paints and surface coatings but also in inks and cleaning products.
Chemical and physical properties of butyl glycol. The basic building blocks of PET are ethylene glycol and terephthalic acid which are combined to form a polymer chain. The resulting spaghetti-like strands of PET are extruded quickly cooled and cut into small pellets.
The resin pellets are then heated to a molten liquid that can be easily extruded or molded into items of practically any shape. PET was first synthesized in North America in. The peaks at 471 ppm peak d and 364 ppm peak h are assigned to the protons from ethylene glycol units in PEF and methylene oxide units in PTMO segments while those at 721 ppm peak c to the aromatic protons from furan rings respectively.
25-Furandicarboxylate with PolyEthylene glycol for Biodegradable Copolyesters with Good Mechanical Properties and Spinnability. Huang L Zhu Z Wu D Gan W Zhu S Li W Tian J Li L Zhou C Lu L. Antibacterial poly ethylene glycol diacrylatechitosan hydrogels enhance mechanical adhesiveness and promote skin regeneration.
More than half of the ethylene oxide produced worldwide is used in the manufacture of monoethylene glycol Occupational Safety and Health Administration 2005. The percentage of total ethylene oxide that is used domestically to manufacture ethylene glycols varies widely between regions. Polysorbates and polyethylene glycol see list in footnote5 and prevail in.
The carcinogenic properties of ethylene oxide are a higher animal health risk for long living animals kept as pet animals than for food producing animals. Therefore the following measures were agreed for feed following the finding of ETO sum of ethylene oxide and 2-chloroethanol expressed as ethylene oxide.