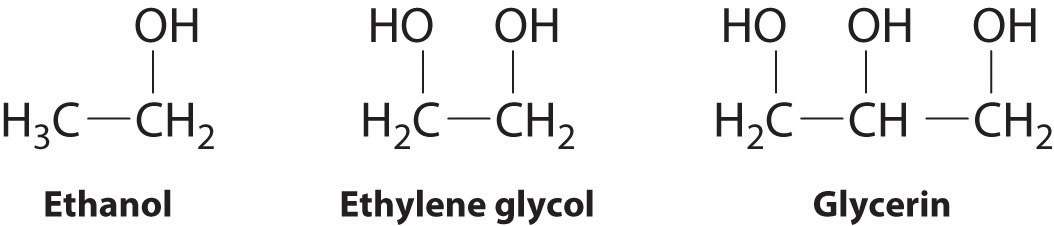
The importance of cellulose has been well known for at least 150 years since it has been used in many fields for daily life applications but only in the last two decades it has been used as biopolymer for biocomposites. B in Figure PageIndex8 most covalent compounds consist of discrete molecules held together by comparatively weak intermolecular forces the forces between molecules even though the atoms within each molecule are held together by strong intramolecular covalent bonds the forces.
Learn About the 3 Types of Intermolecular Forces.
Ethylene glycol kinds of intermolecular forces. Intermolecular forces determine bulk properties such as the melting points of solids and the boiling points of liquids. Liquids boil when the molecules have enough thermal energy to overcome the intermolecular attractive forces that hold them together thereby forming bubbles of vapor within the liquid. Similarly solids melt when the molecules acquire enough thermal energy to overcome the.
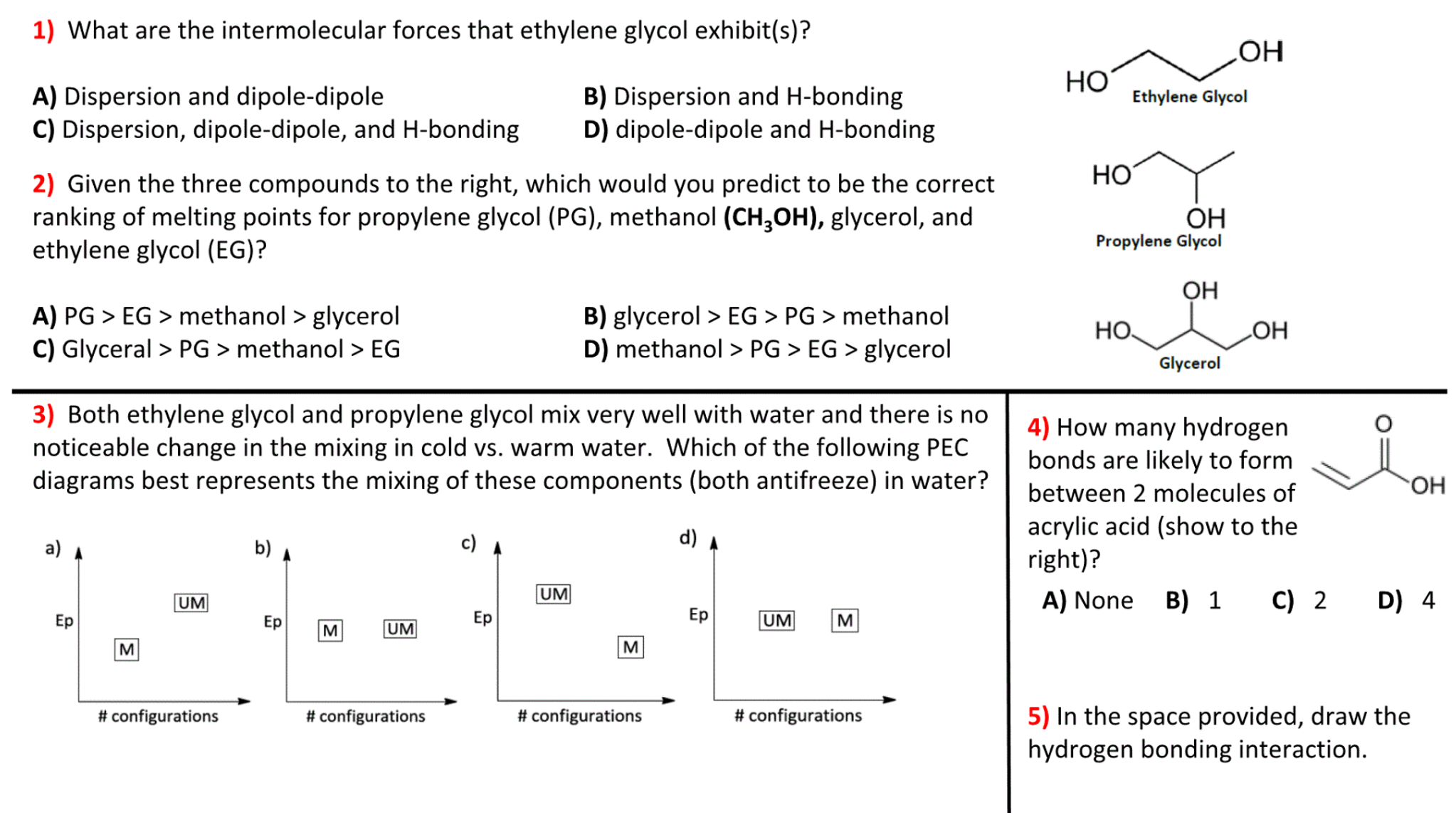
Ethylene glycol is the main ingredient in many antifreeze mixtures for automobile radiators. The two OH groups lead to extensive intermolecular hydrogen bonding. This results in a high boiling point198C.
Thus ethylene glycol does not boil away when it is used as an antifreeze. It is also completely miscible with water. A solution of 60 ethylene glycol in water freezes at 49C 56.
Many kinds of polyethylene are known with most having the. Ethylene is usually produced from petrochemical sources but also is generated by dehydration of ethanol. Polymerization of ethylene to polyethylene is described by the following chemical equation.
N CH 2 CH 2 gas CH 2 CH 2 n solid ΔHn 2571 059 kcalmol 1076 25. Ethylene glycol 1630 349 Glycerol 2110 362 Water. The reason for this is the differences in kinds of polar contributions that give rise to the total cohesive energy densities in each case.
It was mentioned that van der Waals forces result from the additive effects of several different types of component polarities. The inconsistencies in Fig. 1 are due to the fact that while the sum.
Different Kinds of Stimuli-Responsive Hydrogels. Defined by Peppas hydrogels are hydrophilic three-dimension networks which are able to imbibe large amounts of water or biological fluids and thus resemble to a large extent a biological tissueThey are insoluble in any solvent due to the polymer chains being crosslinked by either covalent bonds or physical interactions such as. Learn About the 3 Types of Intermolecular Forces.
What Borax Is How Its Used and Where You Can Find It. Get the Facts About Alum and How Its Used in Everyday Life. Everything You Need to Know About Liquid Nitrogen.
Learn About Molecular and Empirical Formulas and How to Find Them. 10 Interesting Facts About the Element Mercury. Do You Know All.
On the other hand water insolubility of oils and waxes is caused by hydrophobic interaction. 1516 The intermolecular forces between the oil molecules are weaker than the intermolecular bonds between water molecules and the oils are expelled from water to minimize the water-oil interfacial area in the system. This structuring of water at the oil-water interface causes a decrease in entropy.
In an earlier module of this chapter the effect of intermolecular attractive forces on solution formation was discussed. The chemical structures of the solute and solvent dictate the types of forces possible and consequently are important factors in determining solubility. For example under similar conditions the water solubility of oxygen is approximately three times greater than that of.
Academiaedu is a platform for academics to share research papers. Sodium Acetate is chemically designated CH3COONa a hygroscopic powder very soluble in water. Compare the properties of solutions that contain strong electrolytes and weak electrolytes.
The strongest intermolecular forces are dipole-induced dipole weak. Sugar ethyl alcohol a weak electrolyte e. Ethanol sulfuric acid and ethylene glycol popular for use as antifreeze pictured in Figure 6 are examples of liquids that are completely miscible with water.
Two-cycle motor oil is miscible with gasoline. Liquids that mix with water in all proportions are usually polar substances or substances that form hydrogen bonds. For such liquids the dipole-dipole attractions or hydrogen bonding.
Ethylene glycol is the major ingredient in antifreeze. Its condensed structural formula is HOCH. B in Figure PageIndex8 most covalent compounds consist of discrete molecules held together by comparatively weak intermolecular forces the forces between molecules even though the atoms within each molecule are held together by strong intramolecular covalent bonds the forces.
C2h2f2 isomers email protected. We would like to show you a description here but the site wont allow us. Full-density polymers utilize molecular weight and intermolecular forces to provide the strength of the material.
This is partly why PMMA has a high modulus. Other factors such as the lens thickness of the final lens is important too whereas the properties of very low-density polymers 10 of full density 90 air such as porous materials are sensitive to small changes in cross-linking. A molecule is an electrically neutral group of two or more atoms held together by chemical bonds.
Molecules are distinguished from ions by their lack of electrical charge. In quantum physics organic chemistry and biochemistry the distinction from ions is dropped and molecule is often used when referring to polyatomic ions. In the kinetic theory of gases the term molecule is often used.
Interactions between Two Kinds of Gold Nanoclusters and Calf Thymus Deoxyribonucleic Acid. Directions for Preparations to Applications. Zheng-Qi Su Miao-Miao Yin Zi-Qing Yang Ao-Hong Hu and.
Yan-Jun Hu Biomacromolecules 2021 22 11 4738-4747 Article Publication Date Web. Regulated Polyelectrolyte Nanogels for Enzyme. Actually below the CMC concentrations the liquid mixture density and viscosity decrease by the concentration increment due to the fact that the aggregations has not happened yet and subsequently the volume increases or the intermolecular forces decreases.
After micelles formation the volume decreases and the intermolecular forces become stronger. As a result the increment of liquid. Assertion Reason Solid State QNo1 Which of the following is not a characteristic property of Solids.
A Intermolecular distances are short b Intermolecular forces are weak c Constituent particles have fixed positions d Solids oscillate about their mean positions Ansb. Bromine is a liquid and hence it possesses stronger intermolecular forces of attractions than gases. Choice i A mixture of sawdust and water is taken in a Multiple questions 34.
Gases have maximum intermolecular spaces and beaker hence they diffuse in each other forming a homo25. Solids have many free surfaces because the molecules ii A filter paper is folded in the form of a cone. A variety of factors including free volume intermolecular forces chain stiffness and mobility act together to cause this enormous range of transport behavior.
Recent experimental work has provided a great deal of insight while attempts to simulate the diffusional process using molecular mechanics are at a very primitive stage. There is clearly a need for guidance in molecular design of. 1137 a The three molecules have similar structures and experience the same types of intermolecular forces.
As molar mass increases the strength of dispersion forces increases and the boiling points surface tension and viscosities all increase. B Ethylene glycol has an OH group at both ends of the molecule. This greatly increases the.
Pharmaceutics The Science of Dosage Form Design 2Ed MEAulton v. Furthermore it is a semi-crystalline polymer with a high molecular weight that is assembled thanks to the intra-and intermolecular Van der Waals forces. The importance of cellulose has been well known for at least 150 years since it has been used in many fields for daily life applications but only in the last two decades it has been used as biopolymer for biocomposites.
The morphology of. First since e-skin will be exposed to prolonged stresses of various kinds and needs to be conformally adhered to irregularly shaped surfaces materials with intrinsic stretchability and self-healing properties are of great importance. Second tactile sensing capability such as the detection of pressure strain slip force vector and temperature are important for health monitoring in skin.