
How many grams of sodium azide are required to produce 370 L of nitrogen gas at 105 atm and 270 degrees Celsius. No particular concentration limit is specified pending evaluation of the toxicity of the products but.
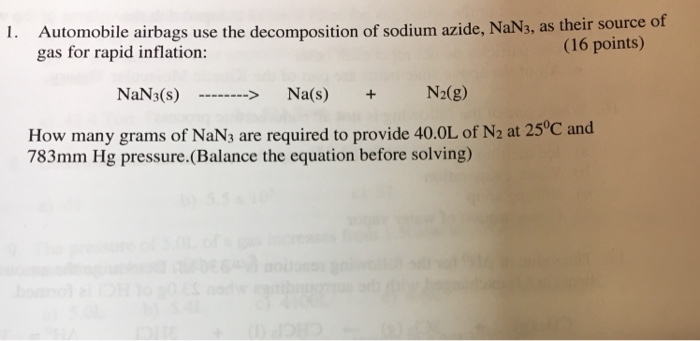
The thermal behavior observed for the decomposition of SeChitosan confirmed the products stability up to temperatures of.
Decomposition sodium azide. Sodium azide is the inorganic compound with the formula NaN 3This colorless salt is the gas-forming component in legacy citation needed car airbag systems. It is used for the preparation of other azide compounds. It is an ionic substance is highly soluble in water and is very acutely poisonous.
Sodium azide in a sample was acidified and the azide was converted to the volatile hydrazoic acid which was trapped in 25 mM sodium hydroxide solution. Determination was performed by isocratic ion chromatography using suppressed conductivity detection. Calibration curves were linear for 05 to 20 ugmL sodium azide and the detection limit was 005 ugmL.
Recoveries of sodium azide from. Sodium azide is made industrially by the reaction of nitrous oxide N 2 O with sodium amide in liquid ammonia as solvent. N 2 O 2 NaNH 2 NaN 3 NaOH NH 3.
Many inorganic azides can be prepared directly or indirectly from sodium azide. For example lead azide used in detonators may be prepared from the metathesis reaction between lead nitrate and sodium azide. The heat from this ignition starts the decomposition of the sodium azide and the generation of nitrogen gas to fill the air bag.
What is particularly amazing is. Sodium azide NaN 3 can decompose at 300 o C to produce sodium metal Na and nitrogen gas N 2. The signal from the deceleration sensor ignites the gas-generator mixture by an electrical impulse creating the high-temperature condition necessary for NaN 3 to decompose.
The nitrogen gas that is generated then fills the airbag. Sodium was first commercially produced by thermal reduction of sodium carbonate with carbon at 1100 degrees Celcius in the Deville process. Pure sodium may be obtained by electrolysis of molten sodium chloride.
It may also be produced by the thermal decomposition of sodium azide. Cite this Article Format. Sodium appears as a silvery soft metal that becomes grayish white upon exposure to air.
Shipped as a solid or molten liquid. Burns violently with explosions that may spatter the material. Used for making gasoline additives electric power cable sodium lamps other chemicals.
Reaction with water to the unstable carbamic acid derivative which will undergo spontaneous decarboxylation. Iodobenzene Dichloride in Combination with Sodium Azide for the Effective Synthesis of Carbamoyl Azides from Aldehydes X-Q. Zhang Synthesis 2008 2589-2593.
Radical Azidonation of Aldehydes L. The balanced equation for the decomposition of sodium azide is _____ 12311. The formula of nitrobenzene is C6H5NO2.
The molecular weight of this compound is _____ amu. The formula weight of ammonium sulfate NH42SO4 rounded to the nearest integer is _____ amu. Substance that dissociates into ions when dissolved in water.
May dissolve in water but it. Add 2 mL of alkali-iodide-azide reagent in the same manner. Stopper the bottle with care to be sure no air is introduced.
Mix the sample by inverting several times. Check for air bubbles. Discard the sample and start over if any are seen.
If oxygen is present a brownish-orange cloud of precipitate or floc will appear. When this floc has settle. However the decomposition of sodium azide is one route to N 2 and decomposition is ammonium dichromate is another.
Both reactions must only be carried out under controlled conditions by a professional. NaN 3 300C 2Na 3N 2 NH 4 2 Cr 2 O 7 N 2 Cr 2 O 3 4H 2 O. Nitrogen is made on massive scale by liquefaction of air and fractional distillation of the resulting liquid air to.
아자이드화 나트륨은 화학식이 NaN 3 인 무기화합물이다. 아자이드화 나트륨은 중요한 의약 중간체로 많은 약품 합성에 사용되며 또한 자동혈구계산기계 방부 살균 자동차 에어백 및. The thermal behavior observed for the decomposition of SeChitosan confirmed the products stability up to temperatures of.
Ameliorative effect of vitamin E and selenium against oxidative stress induced by sodium azide in liver kidney testis and heart of male mice. 2017 91 602 610. Google Scholar Hamza RZ.
Sodium azide decomposes into solid sodium metal and nitrogen gas. How many grams of sodium azide are required to produce 370 L of nitrogen gas at 105 atm and 270 degrees Celsius. NaN3 arrow Na.
Immediately flush eyes with plenty of water for at least 15 minutes occasionally lifting the upper and lower eyelids. Get medical aid imme diately. Get medical aid immediately.
Immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Alkane sulfonate X OSO2R alkyl azide X N3 hemiketal hemiacetal ketal acetal dithiane hydrazone oxime geminal dihalide enol ether enamine ester orthoester nitrile ketene trihalomethyl hydroxamic acid carbamate alkyl haloformate xanthate isocyanate carbodiimide aminal thioester amide urea Summary of Reagents for Oxidative Functional Group Interconversions. Imine organoboranes RCH2BR2.
Chlorine is produced at the positive electrode anode while hydrogen H 2 and sodium hydroxide are produced at the negative electrode cathodeThese three materials are feedstocks for the production of bleach sodium hypochlorite NaOCl and a variety of other products including soda ash Na 2 CO 3. Finally inorganic chemistry is a subject that should not be approached with any degree. Dissolve 10 g sodium azide NaN3 in 40 ml water and add to alkali- iodide solution The resultant reagent should not contain free iodine check through diluting and acidifying and adding starch indicator and observe for blue color Concentrated sulfuric acid Aqueous solution of starch indicator.
Dissolve 2 grams of soluble starch and 02 grams of salicylic acid in 100 ml of hot. Percent composition in chemistry typically refers to the percent each element is of the compounds total mass. The basic equation mass of element mass of compound X 100.
For instance if you had a 800 g sample of a compound that was 200 g element X and 600 g element y then the percent composition of each element would be. This requirement is satisfied in many automotive airbag systems through use of explosive chemical reactions one common choice being the decomposition of sodium azide NaN 3. When sensors in the vehicle detect a collision an electrical current is passed through a carefully measured amount of NaN 3 to initiate its decomposition.
We would like to show you a description here but the site wont allow us. M Thermal decomposition of the fluorocarbon chain in air leads to the formation of oxidized products containing carbon fluorine and oxygen. An index of exposure to these products is possible through their alkaline hydrolysis followed by a quantitative determination of fluoride content.
No particular concentration limit is specified pending evaluation of the toxicity of the products but. Salt concentration 100200 mM. Protein concentration 110 mgml.
Storage at 4C with 10 mM sodium azide or 0005 merthiolate is usually safe over long periods but occasionally proteolytic breakdown occurs. Storage at 20C in PBS in 50 glycerol is usually safe over long periods. 50 glycerol does not freeze at 20C Alternatively store at 70C.
Distilled water 20 mL containing 002 sodium azide was added to 02 g of seaweed samples in filter paper and stoppered funnels. Samples were allowed to stand for 18 h at 4 C. Stoppers were removed from the funnels the residues were weighed and WHC calculated as g of water retainedg of dry sample.
Oil Holding Capacity OHC Commercial virgin olive oil 10 mL was added to 02. Supplied in 10mM sodium HEPES pH 75 150 mM NaCl 2 mgml bovine serum albumin BSA and 50 glycerol. Do not aliquot the antibodies.
Do not aliquot the antibodies.