All salts of Group IA and ammonium are soluble. How many liters of 01 M HCl are required to react with 01 mole of sodium phosphate.
Boiling point - the temperature at which a liquid turns into a gas.
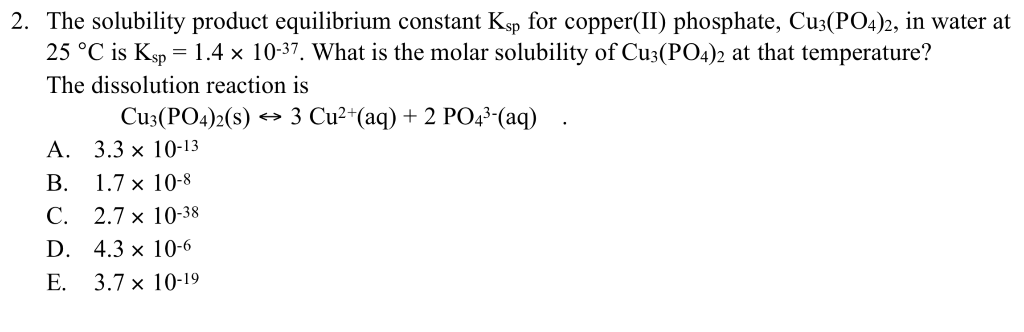
Copper phosphate solubility. Ksp solubility product constants of many popular salts at SolubilityOFthings. Cu 3 PO 4 2. Solubility product constant K sp or the solubility product is the product of the molar concentrations of the constituent ions each raised to the power of its stoichiometric coefficient in the equilibrium equationFor instance if a compound A a B b is in equilibrium with its solution.
SOLUBILITY PRODUCT CONSTANTS The solubility product constant K sp is a useful parameter for calculating the aqueous solubility of sparingly soluble compounds under various conditions. It may be determined by direct measure-ment or calculated from the standard Gibbs energies of formation f G of the species involved at their standard states. Thus if K sp Mm An is the equilibrium.
Solubility Product Constants near 25 C. Ionic Compound Formula K sp. Aluminum hydroxide AlOH 3 1810 5 Aluminum phosphate AlPO 4 6310 19 Barium carbonate BaCO 3 5110 9 Barium chromate BaCrO 4 1210 10 Barium fluoride BaF 2 1010 6 Barium hydroxide BaOH 2 510 3 Barium sulfate BaSO 4 1110 10 Barium sulfite BaSO 3 810 7 Barium thiosulfate BaS 2 O 3.
For ionic compounds with limited solubility in water an equilibrium constant K sp can be defined from the ion concentration in water from the equation. M m A n s mM n aq nA m-aq. Where M m A n is the slightly soluble substance and M n and A m-are the ions produced in solution by dissosiation of M m A n.
K sp M n m A m- n. The table below gives calculated values of K. Solubility of the host mineral within nascent hydrothermal solutions in the source rocks.
Copper is found in association with many other metals and deposit styles. Commonly copper is either formed within sedimentary rocks or associated with igneous rocks. The worlds major copper deposits are formed within the granitic porphyry copper style.
Copper is enriched by processes during. 90 g100 mL 20 C Solubility in ethanol. Insoluble Basicity pK b 16 Structure.
A 1123772 nm b 0810461 nm c 0592271 nm. Irritant Safety data sheet. R-phrases outdated See TfM R36-See TfM R38.
Sodium phosphate Na3PO4 reacts with HCl to form H3PO4 and NaCl. How many liters of 01 M HCl are required to react with 01 mole of sodium phosphate. Write the following into a balanced equation.
A When a mixture of copper II oxide and carbon is heated elemental copper forms and carbon monoxide evolves into the atmosphere. B When a concentrated solution of sodium. Solve for x and youll know how soluble the compound is.
Because of how the solubility constant is defined your answer will be in terms of moles of the compound dissolved per liter of water. You may need a calculator to find the final answer. The following is for solubility in pure water not with any common ions.
7110 9 x2x 2. Cu2O is obtained by oxidizing copper metal or reducing sulfur oxide copperII solutions while CuO is obtained by pyrometallurgical methods used to remove copper from ores. Most of the preservatives in wood are made from copper.
This is often used as a pigment to shape various glazes. Comment on the solubility of cuprous oxide in water. Copper brazing alloys L-ZnCu42 formerly standardized in DIN 813 part 1 and CU301 EN 1044 and the silver solders AG306 and AG304 are preferred for the manganese-containing Cu-Ni alloys.
These brazing alloys together with CU305 and AG203 are used for iron-containing alloys. Only high-silver content brazing alloys should be used for brazed joints at risk of corrosion eg. AG105 AG203 and.
For more Solubility Complete data for COPPERII HYDROXIDE 6 total please visit the HSDB record page. Hazardous Substances Data Bank HSDB 325 Density. The Merck Index - An Encyclopedia of Chemicals Drugs and Biologicals.
Merck and Co Inc 1996 p. Hazardous Substances Data Bank HSDB 326 StabilityShelf. The copper ion is the component of copper sulfate with toxicological implications.
Copper ions appear to bind to functional groups of protein molecules in fungi and algae and cause protein denaturation producing cell damage and leakage. Protein components that act as binding sites are sulfidal groups phosphate thiol carboxyls and. Boiling point - the temperature at which a liquid turns into a gas.
Melting point - the temperature at which a solid turns into a liquid. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen.
Haifa MKP is compatible with most commonly used pesticides and fertilizers. However it should not be mixed with calcium or magnesium fertilizers. Iron manganese zinc and copper must be in the form of chelates.
On young leaves spray concentration of 10 Haifa MKP is recommended in most crops. Barium nitrate ammonium phosphate 11. Calcium hydroxide ironIIIchloride 12.
Rubidium fluoride copperIIsulfate Solubility Rules. All salts of Group IA and ammonium are soluble. All salts of nitrates chlorates and acetates are soluble.
All salts of halides are soluble except those of silverI copperI leadII and mercuryI. All salts of sulfate are soluble. Copper Sulfate Crystal.
Copper sulfate crystals naturally form blue diamonds. These crystals are extremely easy to grow. Simply dissolve copper sulfate into a cup of boiling water until no more will dissolve.
Allow the container to rest undisturbed overnight. Its best to collect the crystals with a spoon or toothpick since touching the solution will turn your skin blue and. Silver phosphate Ag 3 PO 4 is yellow precipitate and dissolve in dilute nitric acid and ammonia.
Barium phosphate Ba 3 PO 4 2 the white precipitate dissolve in HCl. Ferric phosphate - FePO 4 - yellow precipitate - dissolve in HNO 3 and not dissolve in CH 3 COOH. Identify phosphate ion in compounds - qualitative analysis Halide ion precipitates and colours.
Chloride bromide iodide. Reactions catalyzed by phase II enzymes generally increase water solubility and promote the elimination of these compounds. Bipolar disorder a mood disorder previously called manic-depressive illness Bipolar disorder is characterized by severe alterations in mood.
During manic episodes a person may experience extreme elevation in energy level and mood euphoria or extreme. Diammonium hydrogen phosphate is an inorganic phosphate being the diammonium salt of phosphoric acid. The commercially available fertilizer has an analysis of 18-46-0 N-P2O5-K2O and is marketed under the name diammonium phosphate or DAP.
It has a role as a fertilizer. It is an inorganic phosphate and an ammonium salt. Calcium phosphate solubility is 20 mgL.
In steal industries calcium is applied as a blotter and is added to aluminium copper and lead alloys. Calcium can extract sulphur dioxide from industrial exhaust and neutralize sulphuric acids before discharge. Other examples of calcium applications are calcium hypo chloride as bleach and for disinfection calcium phosphate in glass and porcelain.
Solubility Fe Ferrous ammonium phosphate FeNH 4PO 4H 2O Soluble 29 Ferrous ammonium sulfate NH 4SO 4FeSO 46H 2O 14 Iron chelates NaFeEDTA NaFeHPDTA NaFeEDDHA NaFeDTPA FeHEDTA FeEDDHA Soluble Soluble Soluble Soluble Soluble Soluble 5 11 5 9 6 10 5 9 6. Iron polyflavonoids Organically Bound Fe 9 10 Ferrous sulfate FeSO 47H 2O Soluble 20 Ferric sulfate FeSO 4 34H 2O. Because of their high solubility in the aquatic environments heavy metals can be absorbed by living organisms.
Once they enter the food chain large concentrations of heavy metals may accumulate in the human body. If the metals are ingested beyond the permitted concentration they can cause serious health disorders Babel and Kurniawan 2004. Therefore it is necessary to treat metal.
Despite its water solubility cytochrome c is usually bound to the external surface of the IMM due to the interaction with the cardiolipin. This interaction crucial in the determination of the cell fate helps the shuttle to reach its electron acceptor complex IV. Cytochrome c oxidase is the last complex of the electron transport.
Electrons from cytochrome c are accumulated in copper. While this article states Dr. Edwards research as reducing the lead leachrate from 16 times that of the Detroit water to only 4 times with phosphate added I have seen another report that stated.