
Add the NaOH solution dropwise to the copper solution. An acid-base indicator changes its colour depending on the pH eg phenolphthalein.
16 IN 1 WATER TEST KIT Hofun water test strips is equipped with 16 major function including.
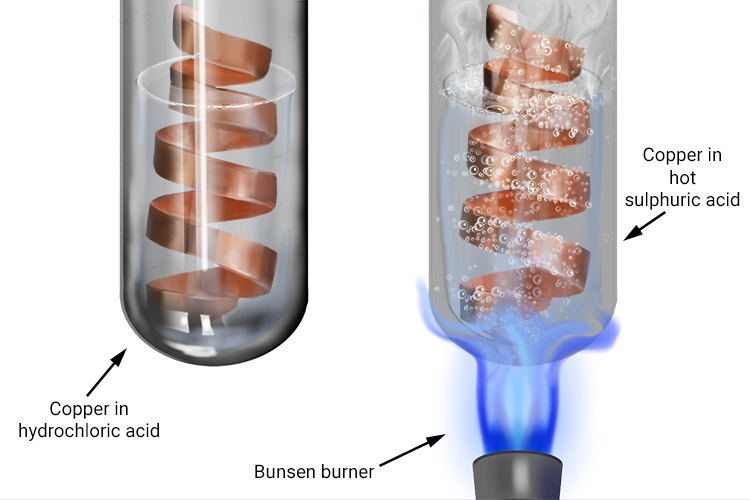
Copper acid or base. When heating the copperII oxide and dilute sulfuric acid avoid boiling off the water and allowing the copper sulfate to appear and then decompose with excessive heating this is unsafe. The sulfur dioxide gases are toxic and can cause breathing difficulties. In the final optional stage of the procedure do not attempt to evaporate the acid to obtain crystals by heating with a Bunsen.
Beetroot acid-base indicators have optical stability at pH 4 to 5 but are structurally unstable at extremes of pH. So the red colour in urine after eating beetroot depends on urine pH and the pigments not being broken down by digestion processes. Eating excess beetroot as in borscht soup usually causes red or pink urine.
Bromocresol purple acid-base indicator 60 Indicator Table Bromocresol. An acid-base indicator changes its colour depending on the pH eg phenolphthalein. Redox indicators are also frequently used.
A drop of indicator solution is added to the titration at the start. At the endpoint has been reached the colour changes. It is an instrument that measures the electrode potential of the solution.
These are used for titrations based on a redox. LME copper stocks also declined overnight providing support to the metals price but tightness in the market has eased with the cashthree-month spread now at 125 per tonne backwardation. The power curtailment in East China has weighed on sulfuric acid demand a by-product from copper smelters and prices have fallen Stablum said.
Base metals prices on the London Metal Exchange rebounded during morning trading on Thursday October 28 with nickel and copper leading the pack higher. The three-month nickel price was recently at 19735 per tonne up by 17 from 19412 per tonne at. Copper can act as a catalyst meaning a substance that can speed up a chemical reaction and improve its efficiency.
It does so by reducing the activation energy. Catalysts in biological reactions are called enzymes. Copper speeds up the reaction between zinc and dilute sulfuric acid.
It is found in some enzymes one of which is involved in. The 3000 m 2 of copper sheet on the Copper Box in Londons Olympic Park is pre-oxidised in the copper factory. The chocolate brown film of copper oxide advances the patination process and provides architects with a different colour option to the bright new copper.
Eventually a film of green copper salts will appear on top of the oxide layer. This will take up to twenty years or more. Solvent TC pK a.
1341 Acetylacetone is a weak acid. C 5 H 8 O 2 C 5 H 7 O 2 H IUPAC recommended pK a values for this equilibrium in aqueous solution at 25 C are 899 004 I 0 883 002 I 01 M NaClO 4 and 900 003 I 10 M NaClO 4. I Ionic.
Nucleic acid analogues are compounds which are analogous structurally similar to naturally occurring RNA and DNA used in medicine and in molecular biology research. Nucleic acids are chains of nucleotides which are composed of three parts. A phosphate backbone a pentose sugar either ribose or deoxyribose and one of four nucleobasesAn analogue may have any of these altered.
Copper oxide ores are leached with sulfuric acid to liberate the copper minerals into a solution of sulfuric acid laden with copper sulfate solution. The copper sulfate solution called the. The addition of 12M sulfuric acid reverses the changes through the copper hydroxide precipate back to clear light blue color of the original solution.
This demo is a good illustration of Le Chateliers principle and of complex ion formation. Allow about 10 minutes for this demo. Two days of lead time is required for this project.
The copper ion in. A strong base is a base that is completely dissociated in an aqueous solution. These compounds ionize in water to yield one or more hydroxide ion OH - per molecule of base.
In contrast a weak base only partially dissociates into its ions in water. Copper oxide dissolves in acid regenerating the copper II ion which once again binds to water. Be careful in handling NaOH for it is a strong base which will sting if it contacts the skin.
Add the NaOH solution dropwise to the copper solution. After a blue precipitate is formed periodically test the acidity of the solution by dipping your stirring rod into the solution and touching. Whereas Cr 3 is hard acid and prefers to be N-bound.
4 The molecule CH 3 2 NCH 2 PF 2 would bond to BF 3 through N whereas it would bond to BH 3 through P. BF 3 is a hard acid and prefers to bind with N atom - a hard base. Whereas BH 3 is a soft acid.
On the other hand the Copper oxide is a base but not an alkali as it neutralizes the acid in aqueous solution but does not dissolve in water. A strong base is a chemical compound that gets deprotonated or removes a proton H from a molecule of a very weak acid in an acid-base reaction. Hydroxides of alkali metals and alkaline earth metals.
Copper equivalent CuEq calculations use metal prices assumptions of 300 lb for copper 110 lb for zinc 100 lb for lead 1300 oz for gold and 1800 oz for silver. Make sure this fits by entering your model number. 16 IN 1 WATER TEST KIT Hofun water test strips is equipped with 16 major function including.
PH Mercury Lead Iron Copper Total Alkalinity Hardness Nitrite NitrateBromine Free Chlorine Total Chlorine Fluride ChromiumCrCarbonate RootCyanuric acid covers main influencing factors which pollute your swimming pool hot tub or. Each of the acidbasesalt circuits requires two centrifugal pumps. Pipes with a length of 100 m from the reservoirs to the cells are required too.
The AB-FB system energy is stored in both salinity and pH gradients. These gradients are constituted by the separation of ions from acid base and salt or water solutions. In this way this system counts with four types of solutions.
Citric acid 1 1. Sugar beet syrup 1 1. Raw sugar syrup 1 1.
1 Tinning is necessary in the drinks and foods industry 2 There is a risk of explosion due to the formation of copper acetylide 3 1 very good. Oxide ores and certain sulfide ores are placed onto a leach pad and saturated with weak sulfuric acid solution that dissolves the copper mineral content. The resulting copper-bearing solution is collected and pumped to a solvent extraction plant.
Smelting and Extraction. The dried copper concentrates are sent to the smelting operation where it is reduced and melted in several. The Chemistry Of Etching Acid.
At Advanced Chemical Etching our preferred etchant etching acid is Ferric Chloride FeCl3. Acid etching metal can be traced back as far as 1531 when the French scholar Jehan le Begue wrote a recipe for acid etching on iron formulating acid for etching by distilling ammonium chloride ordinary alum and ferrous sulphate in a mixture of water and vinegar. Copper is an essential trace element that is included in some over-the-counter multivitamin and mineral supplements even though copper deficiency is quite rare and supplementation is rarely needed.
The amounts of copper found in typical supplements has not been associated with serum enzyme elevations or with clinically apparent liver injury. However accidental or intentional copper overdose. Upper Saddle River NJ.
Impact of Learners Prior Knowledge on Their Use of Chemistry Computer Simulations. A Case Study Journal of Science Education and Technology 17 466-482. Schroeder JD and Greenbowe TJ.
Polyhedron publishes original fundamental experimental and theoretical work of the highest quality in all the major areas of inorganic chemistry. This includes synthetic chemistry coordination chemistry organometallic chemistry bioinorganic chemistry and solid-state and materials chemistry. In this novel acid-base jointly promoted formation of 123-triazoles HOAc was recognized to accelerate the conversions of the C-Cu bond-containing intermediates and buffer the basicity of DIPEA.
As a result all drawbacks occurring in the popular catalytic system CuINR 3 were overcome easily. Chem 2011 76 6832-6836.