Pyrometallurgical techniques use heat to separate copper from copper sulfide ore concentrates. When copperII sulfate pentahydrate CuSO 4 5H 2 O is heated it decomposes to the dehydrated form.
An adult human needs around 12 milligrams of copper a day to help enzymes transfer energy in cells.
Copper 2 sulfate with water heat. Copper sulfate may refer to. CopperII sulfate CuSO 4 a common compound used as a fungicide and herbicide. CopperI sulfate Cu 2 SO 4 an unstable compound which is not commonly used This page was last edited on 10 April 2021 at 1533 UTC.
A tolerance of 1 ppm is established in potable water for residues of copper resulting from the use of the algicides or herbicides basic copper carbonate malachite copper sulfate copper monoethanolamine and copper triethanolamine to control aquatic plants in reservoirs lakes ponds irrigation ditches and other potential sources of potable water. Iron forms 2 types of ions namely Fe2 and Fe3. We shall use stoichiometric principles to determine which of these ions is formed in the reaction between iron and copper II sulfate solution.
If Fe2 is formed then equation 1 is correct while equation 2 is correct if Fe3 is formed. Your task is to find out which. Copper sulfate injected ip at 2 mg copperkg into vitamin e selenium deficient rats caused a 6 fold increase in the formation of the lipid peroxidation product ethane caused acute mortality in 45 rats.
Copper II Sulfate anhydrous 7758-98-7 99 Section 4. First Aid Measures Always seek professional medical attention after first aid measures are provided. Immediately flush eyes with excess water for 15 minutes lifting lower and upper eyelids occasionally.
Immediately flush skin with excess water for 15 minutes while removing contaminated clothing. According to the Royal Society of Chemistry copper sulfate becomes dehydrated and changes physical properties when it is heated. The color as well as the makeup of the element changes when heat is applied.
From Gilbert Baker to. When copper sulfate CuSO4 reacts with water H2O the product is still copper sulfate. However it is the hydrated form of the salt and has 5 water molecules.
Thus the product is called copper sulfate pentahydrate. Why is CuSO4 blue. In hydrated CuSO4 the water molecules surrounding the Central metal Cu function as ligands which bring d-d transition and hence emits blue colour in visible.
In this experiment a known mass of hydrated copperII sulfate is heated to remove the water of crystallisation. The mass of water is found by weighing before and after heating. This information is used to find x in the formula CuSO 4xH 2 O using mole calculations.
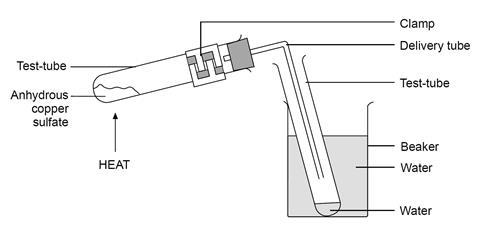
This is a class experiment suitable for students who already have a reasonable understanding of the mole concept. Heat the blue copperII sulfate until it has turned white. Move the flame along the length of the test tube from time to time avoiding the clamp to prevent water condensing on the cooler regions and then running down on to the hot solid possibly cracking the test tube.
Do not heat too strongly nor allow the white colour to darken as the copper sulfate may decompose to produce toxic. 2O copper II sulfate pentahydrate. The water in these compounds can be removed quantitatively by heating the compound with a bunsen burner.
The loss in mass of the compound after losing the water can be used to calculate the amount of water originally in the hydrated sample. When all the water has been removed the ionic compound is said to be anyhydrous. Once a true hydrate had been heated.
Copper sulfate is highly corrosive to plain steel iron and galvanized pipes. It should not be stored in metal containers. Burning copper sulfate may produce irritating or poisonous gases and pollution may be caused by runoff from fire control or dilution water.
Copper sulfate is a strong irritant. There have been reports of human suicide. When copperII sulfate pentahydrate CuSO 4 5H 2 O is heated it decomposes to the dehydrated form.
The waters of hydration are released from the solid crystal and form water vapor. The hydrated form is medium blue and the dehydrated solid is light blue. The balanced equation is.
CuSO 4 5H 2 Os heat CuSO 4 s 5H 2 Og. Product Name CopperII sulfate Cat No. AC422875000 CAS-No 7758-98-7 Synonyms Cupric sulfate anhydrous.
Copper monosulfate Recommended Use Laboratory chemicals. Uses advised against Food drug pesticide or biocidal product use. Details of the supplier of the safety data sheet.
The treatment technique regulation for lead referred to as the Lead and Copper Rule requires water systems to control the corrosivity of the water. The regulation also requires systems to collect tap samples from sites served by the system that are more likely to have plumbing materials containing lead. If more than 10 percent of tap water samples exceed the lead action level of 15 parts.
As barium sulfate has a high melting point and is insoluble in water it is used as a release material in casting of copper anode plates. The anode plates are cast in copper molds so to avoid the direct contact of the liquid copper with the solid copper mold a suspension of fine barium sulfate powder in water is used as a coating on the. In this experiment the hydrates of copperII sulfate CuSO.
O and magnesium sulfate MgSO. O will be studied. The water molecules are loosely attached to the salt and can be removed upon heating yielding the anhydrous salt salt without water.
The mass of water lost during heating can be determined and the. Heat the copper sulfate solution to evaporate half of the water. Pour the solution into a watch glass and leave to allow all of the water to evaporate.
Record the appearance of the copper. Sr 2 aq 2OH-aq Note that while calcium hydroxide barium hydroxide and strontium hydroxide are strong bases they are not very soluble in water. The small amount of compound that dissolves dissociates into ions but most of the compound remains a solid.
Hot process semi-transparent patina. Heat metal and apply a fresh mixture for each coloring. Solution hot 180 to 190F metal hot 200F cold wash water applied after metal has cooled to around 100F.
Wash solution over metal surface let. Copper sulfate is used widely as an agricultural poison and as an algicide in water purification. Copper compounds such as Fehlings solution are used in chemical tests for sugar detection.
Copper is an essential element. An adult human needs around 12 milligrams of copper a day to help enzymes transfer energy in cells. Excess copper is toxic.
Genetic diseases such as. Around in the beaker until the copper has completely dissolved. Add water until the beaker is about half full.
The remainder of the experiment can be carried out at your lab bench. Record in your notebook a description of everything you see in this step. Background information The reaction that takes place can be written two different ways.
Here is the first. 3Cus 8HNO 3 aq 3CuNO 3 2. Copper is believed to have been used first by Neolithic man as a substitute for stone around 8000 BC.
The science of metallurgy emerged when copper was heated and mold-casted into shapes in Egypt around 4000 BC. In 3500 BC fire and charcoal were used to smelt ores and copper was alloyed with tin to create bronze giving. It is then converted to copper II hydroxide CuOH 2 by reaction with base.
When this compound is heated it is transformed to copper II oxide CuO. Copper II oxide is then reacted with acid to form copper II sulfate CuSO 4. Finally the copper ions in the copper sulfate are reduced to copper metal by magnesium.
Pyrometallurgical techniques use heat to separate copper from copper sulfide ore concentrates. Process steps include mining concentration roasting smelting converting and finally fire and electrolytic refining. 1232 Process Description2-4 Mining produces ores with less than 1 percent copper.
Concentration is accomplished at the mine sites by crushing grinding and flotation. CuSO 4 5 H 2 Os HEAT — CuSO 4 s 5 H 2 O g hydrate anhydrate. Experimentally measuring the percent water in a hydrate involves first heating a known mass of the hydrate to remove the waters of hydration and then measuring the mass of the anhydrate remaining.
The difference between the two masses is the mass of water lost. Dividing the mass of the water lost by the original mass.