
Hydrogen sulfide is a. Halitosis is very common in general population and nearly more than 50 of the general population have halitosis.
Chlorine however has some undesirable by-products and can leave chlorine tastes and odors.
Common uses for hydrogen sulfide. Hydrogen sulfide is often produced from the microbial breakdown of organic matter in the absence of oxygen such as in swamps and sewers. This process is commonly known as anaerobic digestion which is done by sulfate-reducing microorganisms. H 2 S also occurs in volcanic gases natural gas and in some sources of well water.
The human body produces small amounts of H 2 S and uses it as a. Hydrogen sulphide gas is also produced by heating sulfur with solid organic compounds and by reducing sulfurated organic compounds with hydrogen. It is used for the detection of cations which is studied in analytical chemistry.
Hydrogen sulfide is a precursor to elemental sulfur. Several organosulfur compounds such as. Hydrogen sulfide can also result from industrial activities such as food processing coke ovens kraft paper mills tanneries and petroleum refineriesHydrogen sulfide is a flammable colorless gas with a characteristic odor of rotten eggs.
It is commonly known as hydrosulfuric acid sewer gas and stink damp. People can smell it at low levels. Research suggests that diarrhea may be more common in hydrogen sulfide SIBO 1 2 3 though this has been disputed 16 Trusted Source PubMed Go to source.
There is a view held by some individuals and practitioners that more specialized treatments specifically targeting hydrogen sulfide like low sulfur diets and avoiding sulfuric medications will help people with this type of SIBO to get. Added chlorine quickly oxidizes sulfide hydrogen sulfide and bisulfide to form compounds that do not cause foul tastes or odors in drinking water. Yellow sulfur particles may also be produced which should be filtered out by a fine-retention sediment filter.
Otherwise they can form a yellow film on clothing and fixtures. However this process may produce an objectionable taste due to excess. Hydrogen Sulfide H 2 S is a naturally occurring gas found in crude oil natural gas volcanic gases and hot springs.
It can also be generated by the bacterial breakdown of organic matter the processing of municipal wastewater as well as a multitude of manufacturing and industrial processes. Hydrogen sulfide is a colorless gas with a distinctive foul odor of rotten eggs. Depending on the.
Common Uses for Barium. Barium Sulfate in Oil Production. Barium Sulfate is primarily used when drilling for new oil wells which is by far the most common use for bariumIt is combined with water and some other minerals to create drilling mud.
This mud gets pumped into the drilling holes and because of its weight it helps to prevent the oil from exploding out into the environment. Sulfide British English also sulphide is an inorganic anion of sulfur with the chemical formula S 2 or a compound containing one or more S 2 ions. Solutions of sulfide salts are corrosive.
Sulfide also refers to chemical compounds large families of inorganic and organic compounds eg. Lead sulfide and dimethyl sulfide. Hydrogen sulfide H 2 S and bisulfide SH are the conjugate.
The hydrogen sulfide producing bacteria H 2 S comprise various groups of bacteria and archaea that obtain energy by reducing various compounds that possess sulfur in their molecule including organic compounds sulfur amino acids and inorganic compounds with oxidized sulfur such as sulfate sulfite thiosulfate tetrathionates or elemental sulfur to H 2 S. Hydrogen sulfide is a. The Common Uses of Lithium-Ion Batteries.
January 7 2019 TheEarthAwards Lithium. Not to mention its toxic byproducts when exposed to hydrogen sulfide compounds. Throughout most of the 70s and the 80s various scientists and engineers pioneered and perfected the lithium battery.
In 1979 scientists John Goodenough Ned A. Godshall etal and Koichi Mizushima in separate attempts. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structureThe chemical symbol for Hydrogen is H.
With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table. Its monatomic form H is the most abundant chemical substance in the Universe constituting roughly 75 of all baryonic mass. The use of steel equipment in conjunction with hydrogen sulfide or volatile sulfide compounds will cause it to spontaneously explode in air.
Saxs Dangerous Properties of Industrial Materials. Van Nostrand Reinhold 1996 p. Hazardous Substances Data Bank HSDB Violent reaction with lithium when heated above 260 C.
The most common problem with exposure is that inhaling the dust can cause respiratory irritation particularly in children. Ingesting large amounts of borax can cause nausea vomiting and diarrhea. The European Union EU Canada and Indonesia consider borax and boric acid exposure a potential health risk primarily because people are exposed to it from many sources in their diet and.
Adding manganese which has a greater affinity for sulfur than does iron converts the low-melting iron sulfide in steel to high-melting manganese sulfide. Produced without manganese steel breaks up when hot-rolled or forged. Steels generally contain less than 1 percent manganese.
Manganese steel is used for very rugged service. Containing 1114 percent manganese it provides a hard wear. Polyphenylene sulfide PPS 5090 110 3845 machine parts appliances electrical equipment cellulose diacetate.
1565 670 15 photographic film polycaprolactam nylon 6 40170 30300 1028 bearings pulleys gears Thermosets Heterochain. TraPPE-Explicit Hydrogen is a more complex representation that uses independent interaction sites for all hydrogen atoms as well as some lone pair electrons and bond centers. TraPPE-Small places interaction sites at atomic positions and for some modesl also uses off-center sites to describe technologically important small molecules.
Glow plugs and heat sensors - less common. - Hydrogen Sulfide - Ammonia - Chlorine. Has developed and patented the technology for a wide array of very low cost hydrogen gas sensors.
These chemically active or chemochromic materials form the basis novel thin films or smart coatings that change the hazardous gas safety paradigm by warning of an incipient leak. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. The chemical symbol for Hydrogen is H.
With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table. Its monatomic form H is the most abundant chemical substance in the Universe constituting roughly 75 of all baryonic mass. The most common uses of biofiltration bioscrubbers and biooxidizers include in the processing of waste water capture of VOCs in surface runoff and microbiotic oxidization of air contaminants.
Another way to treat hydrocarbons would be with a Vapor Combustion Unit. A Vapor Combustor Unit VCU is an abatement system used for the destruction of volatile liquid hydrocarbons. Hydrogen sulfide can also quickly foul and ruin water softeners and filter systems.
Aeration andor chlorine treatment utilizing common household laundry bleach has been the most common method in the past to take care of smells from well water. Chlorine however has some undesirable by-products and can leave chlorine tastes and odors. If the pH of the water is over 75 chlorine is not very.
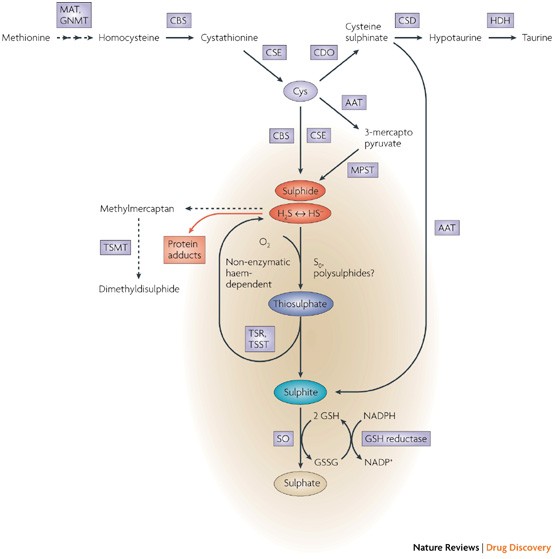
Halitosis is very common in general population and nearly more than 50 of the general population have halitosis. These compounds are mainly hydrogen sulfide and methyl mercaptan. They produce bacteria by enzymatic reactions of sulfur-containing amino acids which are L-cysteine and L-methionine Table 2.
In addition some of the bacteria produce hydrogen sulfide and methyl mercaptan. Iron sulfide hydrogen chloride — iron chloride and hydrogen sulfide poisonous gas lead nitrate potassium iodide — lead iodide and potassium nitrate saltpeter sodium bicarbonate baking soda vinegar — carbonic acid and sodium acetate. Sulfuric acid barium hydroxide — barium sulfate and water.
Silver nitrate sodium chloride — silver chloride and sodium nitrate. Hydrogen Sulfide Contamination and Treatment. Well Water Treatment for Sand and Sediment.
Treatment for Hard Water Contamination. Water Treatment for Corrosivity. PROUD GOLD WINNERS OF THE 2021 AWARD.
BEST OF THE 603. NEW HAMPSHIRE AND MASSACHUSETTS WATER EXPERTS.