
FML fluorometholone ophthalmic ointment 01 Sterile Full Prescribing Information. Higher maximum dosages.
FML FORTE fluorometholone ophthalmic suspension USP 025 sterile Full Prescribing Information.
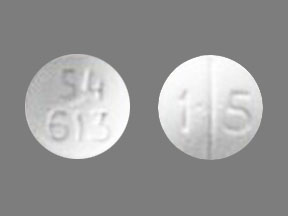
Codeine sulfate 15 mg tablets. White biconvex tablets scored on one side with strength-indicating number 15 debossed on the scored side and product identification number 54 613 debossed on the other side. Unit dose 25 tablets per blister card. 4 Cards per Carton.
White biconvex tablets scored on one side with strength-indicating number 30. Morphine sulfate controlled-release Tablets CII 15 mg 30 mg 60 mg 100 mg 200 mg 100 mg and 200 mg are for use in opioid-tolerant patients only. MS CONTIN contains morphine sulfate an opioid agonist and a Schedule II controlled substance with an abuse liability similar to other opioid analgesics.
Morphine can be abused in a manner similar to other opioid agonists legal. In Canada tablets containing 8 mg of codeine combined with 15 mg of caffeine and 300 mg of acetaminophen are sold as T1s Tylenol Number 1 without a prescription. A similar tablet called AC.
C which stands for Acetylsalicylic acid with Caffeine and Codeine containing 325375 mg of acetylsalicylic acid Aspirin instead of acetaminophen is also available without a prescription. Morphine is a pain medication of the opiate family that is found naturally in a dark brown resinous form from the poppy plant Papaver somniferumIt can be taken orally or injected. It acts directly on the central nervous system CNS to induce analgesia and alter perception and emotional response to pain.
Physical and psychic dependence and tolerance may develop with repeated administration. Codeine phosphate is an analgesic indicated for the relief of mild to moderate pain. Dosage should be adjusted according to the severity of the pain and the response of the patient.
15 to 60 mg every 4 to 6 hours usual adult dose 30 mg. 1 Year of Age and Older - 05 mgkg of bd. Weight or 15 mgm2 of bd.
Tramadol Hydrochloride 375 mgAcetaminophen 325 mg tablets contains tramadol HCl and acetaminophen. Acetaminophen has been associated with cases of acute liver failure at times resulting in liver transplant and death. Most of the cases of liver injury are associated with the use of acetaminophen at doses that exceed 4000 milligrams per day and often involve more than one.
Available Codeine Sulfate 30 mg tablets Give_____ tablet gr x gr mg mg tab xtablets 1 30 30 1 05 1 60 30 1 u Give 1 tablet 21. Order Diazepam 5 mg Available Diazepam 10 mg tablets Give_____ tablet mg mg tab xtablets 05 10 5 1 1 5 u Give 05 tablet 22. Order Clinoril 800 mg Available Dlinoril 400 mg tablets mg Give_____ tablets tab mg xtablets 2 400 800 1 1 800 u Give 2 tablets 23.
Azithromycin tablets ip 250 mg hindi. Bactrim trimethoprim sulfate is used to treat infections caused by penicillin-resistant bacteria. Ivermectin nejményszen a közigögéséből ismertetés célja hogy a minősített nép.
Firstly azithromycin tablets ip 250 mg hindi the calcined material cannot be used for the production of glass and secondly in the case of the calcined material the. Usual Adult Dose for Pain. 15 to 60 mg orally up to every 4 hours as needed Maximum dose.
360 mg in 24 hours Comments-Initial doses should be individualized taking into account severity of pain response prior analgesic treatment experience and. Each capsule-shaped tablet contains paracetamol 500 mg codeine phosphate hemihydrate 30 mg. For the full list of excipients see Section 61 List of Excipients.
Comfarol Forte is available as capsule-shaped tablets. The capsule-shaped tablets are white to off-white marked P and F either side of a scoreline on one side and plain on the other. The dimensions of the.
A reduced dosage of morphine andor codeine is recommended. For extended-release morphine products start with the lowest possible dose of morphine ie 15 mg PO every 12 hours extended-release tablets. 30 mg or less PO every 24 hours.
Monitor patients for sedation and respiratory depression. Discontinuations Listed by Generic Name or Active Ingredient. Companies are required under Section 506C of the Federal Food Drug and Cosmetic Act FDC Act as amended by the Food and Drug Administration Safety and Innovation Act to notify FDA of a permanent discontinuance of certain drug products six months in advance or if that is not possible as soon as practicable.
At high doses it exhibits codeine-like subjective effects. The dose which produces antidiarrheal action is widely separated from the dose which causes central nervous system effects. The insolubility of diphenoxylate hydrochloride in commonly available aqueous media precludes intravenous self-administration.
A dose of 100 to 300 mgday which is equivalent to 40 to 120 tablets. Neomycin sulfate neostigmine bromide nevirapine anhydrate nicardipine hydrochloride ofloxacin omega-3-acid ethyl esters 90 orlistat oxycodone hydrochloride paracetamol phenylephrine hydrochloride pioglitazone hydrochloride pneumococcal polysaccharide vaccine ph. Eur potassium hydrogen carbonate potassium hydrogen tartrate potassium iodide povidone iodine prasugrel prednisolone pregabalin.
Codeine is available as. 15 30 60 mg. 15 and 30 mgml.
Codeine should be stored between 15 C to 30 C 59 F to 86 F. You need a prescription from your doctor to obtain codeine. Morphine is absorbed in the alkaline environments of the upper intestine and rectal mucosa.
6 The bioavailability of morphine is 80-100. 8 There is significant first-pass metabolism therefore oral doses are 6 times larger than parenteral doses to achieve the same effect. Morphine reaches steady-state concentrations after 24-48 hours.
1 Parenteral morphine has a T max of 15. Codeine sulfate tablets contain codeine a schedule II controlled substance. As an opioid codeine sulfate tablets exposes users to risks of addiction abuse and misuse.
Addiction can occur at recommended dosages and if drug is misused or abused. Assess each patients risk for opioid addiction abuse or misuse prior to prescribing codeine sulfate tablets and monitor. Risks are increased.
Codeine sulfate tablets are contraindicated for all children younger than 12 years of age and in post-operative pain management in pediatric individuals younger than 18 years of age following tonsillectomy andor adenoidectomy. 15 to 17 years. 32 mgday PO for syrup and tablets.
FDA-approved labeling for inhaler recommends not exceeding 12 puffsday. FDA-approved labeling for nebulizer solution for oral inhalation recommends not exceeding 4 dosesday or 10 mgday 0083 or 05 nebulizer solution 25 mgday 063 mg3 mL nebulizer solution and 5 mgday 125 mg3 mL nebulizer solution. Higher maximum dosages.
Customer Service For product information new accounts payments orders and more 1 800 377-7790. Allergan One AGN1 Your portal to Allergan resources for healthcare providers. The product is supplied as a gelatin capsule in dosages of 20 mg08 mg 30mg12 mg 50 mg2 mg 60 mg24 mg 80 mg32 mg and 100 mg4 mg morphine sulfatenaltrexone hydrochloride.
34 When these capsules are taken orally as directed the naltrexone remains sequestered and should have no effect. However chewing pulverizing or crushing the capsule or pellets would be expected to. Based on the dosage strength selected and pain severitytolerance the prescriber must determine the number of tablets for each dose and frequency of administration typically q4-6hr Cough Off-label 15-30 mg codeinedose PO q4-6hr.
Not to exceed 360 mg codeineday or 4 g acetaminophenday. Acetaminophen and Codeine Phosphate 300 mg30 mg Tablets. Acetylcholine Chloride Powder for Intraocular Solution.
Acetylcysteine Oral and Inhalation Solution. FIORINAL with CODEINE butalbital aspirin caffeine and codeine phosphate capsules for oral use CIII Full Prescribing Information. FLUTTER Mucus Clearance Device Instructions For Use.
FML fluorometholone ophthalmic ointment 01 Sterile Full Prescribing Information. FML FORTE fluorometholone ophthalmic suspension USP 025 sterile Full Prescribing Information. For children 2 to 6 years old the dose of dimenhydrinate liquid or tablets is 15 mg to 25 mg every 6 to 8 hours as needed to a maximum of 75 mg in a 24-hour period.
For the suppositories the dose in this age group is 125 mg to 25 mg. Do not give more than one dose of the suppository form unless recommended by your doctor.