This complex is featured with an ironI center which bonds to a 12-ethanediamine en. As the carbonate reacts with acids in the soil to produce bicarbonate and eventually leading to the production of CO2 and water.
It is a fire hazard but is safe if stored in sealed bags and well away from combustible organic matter.
Co2 reduction ammonium bicarbonate. The control of arterial CO2 tension by central nervous system and respiratory system and control of plasma bicarbonate by kidneys stabilize the arterial pH by excretion or retention of acid and alkali. This balance is represented by the Henderson-Hassalbalch equation given by. Where HCO3- represents in the plasma bicarbonate concentration and pCO2 is.
Sodium bicarbonate is a medication used in the management and treatment of multiple disease pathologies. It is a general chemical compound by classification. This activity outlines and reviews the indications action and contraindications for sodium bicarbonate as a valuable agent in the treatment management and therapy of lactic acidosis QRS prolongation and other disorders when.
To explore alternative approaches to the CO2 reduction to formate and provide an insight into the spin state effect on the CO2 reduction we theoretically designed a kind of low-valence ironI model complex whose doublet quartet and sextet states are denoted as 2FeI 4FeI and 6FeI respectively. This complex is featured with an ironI center which bonds to a 12-ethanediamine en. Ammonium nitrate 335345 N.
This is a very widely used fertiliser for top-dressing. Half the nitrogen as nitrate is very readily available. It is marketed in a special prilled or granular form to resist moisture absorption.
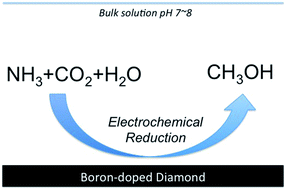
It is a fire hazard but is safe if stored in sealed bags and well away from combustible organic matter. Because of the ammonium present it has an acidifying effect. Electrochemical conversion of CO2 into value-added chemicals holds promise to enable the transition to carbon neutrality.
Enhancing selectivity for a specific hydrocarbon product is challenging. Expectorant in cough syrups. The ammonium ion NH4 in the body plays an important role in the maintenance of acid-base balance.
The kidney uses ammonium NH4 in place of sodium Na to combine with fixed anions in maintaining acid-base balance especially as a homeostatic compensatory mechanism in metabolic acidosis. The therapeutic effects of Ammonium Chloride depend upon the. Here we report reduction of carbon dioxide to C2 products acetic acid and ethanol over a 3D dendritic copper-cuprous oxide composite fabricated by in situ reduction of an electrodeposited copper complex.
In potassium chloride aqueous electrolyte the applied potential was as low as -04 V vs reversible hydrogen electrode the overpotential is only 053 V for acetic acid and 048 V for. When the equation o2 c5h12 o2 co2 h2o is balanced the coefficient of o2 is. CO2 can be used to flood the surgical field during cardiac surgery.
Because of its density carbon dioxide displaces the air surrounding the open heart so that any gas bubbles trapped in the heart are carbon dioxide rather than insoluble nitrogenSimilarly CO2 is used to de-bubble cardiopulmonary bypass and extracorporeal membrane oxygenation ECMO circuits. Final urine P CO2 may therefore rise above blood P CO2 as H and HCO 3 react and come to equilibrium with CO 2 and be an index of distal nephron H secretion. Regulation of Acid-Base Transporters in the Distal Nephron.
Regulation of distal nephron acid-base transport is crucial because this is the final site of control of urine composition. The distal tubule responds to systemic. Acid-base homeostasis and pH regulation are critical for both normal physiology and cell metabolism and function.
The importance of this regulation is evidenced by a variety of physiologic derangements that occur when plasma pH is either high or low. The kidneys have the predominant role in regulating the systemic bicarbonate concentration and hence the metabolic component of acid-base. Ammonia is a compound of nitrogen and hydrogen with the formula NH 3A stable binary hydride and the simplest pnictogen hydride ammonia is a colourless gas with a distinct pungent smell.
It is a common nitrogenous waste particularly among aquatic organisms and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to 45 percent of the world. 6 CO2 g 6 H2 O l sunlight 6 O2 g C6 H12 O6 aq Suppose you determine that a particular plant consumes 378 g of CO2 in one week. Assuming that there is more than enough water present to react with all of the CO 2 what mass of glucose in grams can the plant synthesize from the CO 2.
258 g C6 H12 O6. Magnesium hydroxide the active ingredient in milk of magnesia neutralizes. The gel bands were dried in a speedvac before adding 100 mM DTT in 50 mM ammonium bicarbonate for 1 h reduction at room temperature.
If ammonium reabsorbed was not excreted in the urine. Hypercapnia determines a reduction of pendrin expression by up to 50 contributing to the increased plasma bicarbonate and decreased plasma chloride observed in chronic respiratory acidosis 11 17. Pendrin localized in the cellular apical membrane of cortical collecting ducts and connecting tubules acts as a Cl.
As the carbonate reacts with acids in the soil to produce bicarbonate and eventually leading to the production of CO2 and water. Urea application Adding urea to soils for fertilisation leads to a loss of the CO2 that was fixed during the manufacturing process. Similar to the reaction following the addition of lime the bicarbonate that is formed evolves into CO2 and water.
0 1000 2000. Sodium carbonate and hydrochloric acid reaction type. Metabolic disorders involve a primary change in the serum bicarbonate andor anion gap.
Respiratory disorders involve primary changes in the pCO2 due to changes in CO2 removal by the lungs. ABGVBG isnt needed to evaluate metabolic pH disorders. Complete analysis of pH status requires blood gas analysis but all you need to determine the metabolic pH disorders is an electrolyte panel.
Its reduction potential E 0 420 mV is low enough to reduce NAD the main electron carrier in the cell E 0 280 mV under physiological conditions in E. Coli Huang et al 2012. Another advantage is that it can be electrochemically produced from renewable sources Yishai et al 2016 and is seen as a promising path for carbon negative biomass formation.
How many moles of Co2 are present in 0150 L of a 0200 M solution of CoI2. 11 ratio2 M x mol 150L. How many milliliters of a stock solution of 121 M HNO3 would be needed to prepare 0500 L of 0500 M HNO3.
ML ML 121M xL 5M 5L 0207 in mL 207mL. Calculate the molarity of each of the following solutions. 435 mol of LiCl in 320 L solution.
435 mol 32 L 136 M. The Solvay process or ammonia-soda process is the major industrial process for the production of sodium carbonate soda ash Na 2 CO 3The ammonia-soda process was developed into its modern form by the Belgian chemist Ernest Solvay during the 1860s. The ingredients for this are readily available and inexpensive.
Salt brine from inland sources or from the sea and limestone from quarries. A Ammonium carbonate NH42CO3 CAS Reg. 8000-73-5 is a mixture of ammonium bicarbonate NH4HCO3 and ammonium carbamate NH2COONH4.
It is prepared by the sublimation of a mixture of ammonium sulfate and calcium carbonate and occurs as a white powder or a hard white or translucent mass. Which one of the following is a molecular Aim To detect one cation and one anion in the given salt from the following ions. Cations -Pb2 Cu2 As3 Al3 Fe3 Mn2 Ni2 Zn2 Co2 Ca2 Sr2 Ba2 Mg2 NH 42 2 2 3 2Anions -CO S2 SO SO NO NO Cl Br I PO CO 3 3 423 424 CHCOO.
CrO2 Figure 1 shows the normalized noise spectral density. Northern Arizona University and Raymond Chang this success guide is written for use with General Chemistry. It aims to help students hone their analytical and problem-solving skills by presenting detailed approaches to solving chemical problems.
The transformation of CO2 into a precipitated mineral carbonate through an ex situ mineral carbonation route is considered a promising option for carbon capture and storage CCS since i the captured CO2 can be stored permanently and ii industrial wastes ie coal fly ash steel and stainless-steel slags and cement and lime kiln dusts can be recycled and converted into value-added.